Figure 2. Pluripotency analysis of human pluripotent cells by embryoid body and directed differentiation.
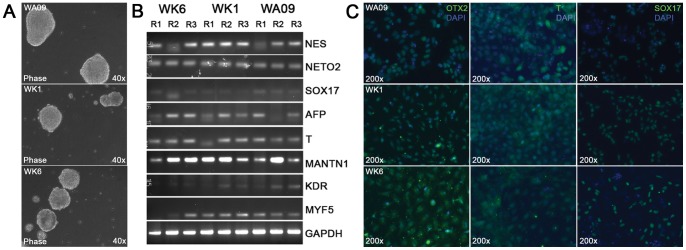
(A) Spontaneous differentiation of human pluripotent stem cells through EB formation. Pluripotent stem cells (WA09, WK1 and WK6) were seeded onto ultra-low binding substrates (Becton Dickinson) and assayed for aggregation (see experimental procedures). Human iPS cells aggregated to EBs with a tempo and size comparable to EBs derived from WA09 hESCs. (B) Analysis by semi-quantitative RT-PCR of germ layer marker expression by EBs derived from WA09, WK1 and WK6 cells. EB populations were grown in triplicates and harvested after 21 days of aggregation for the preparation of single stranded cDNA from total RNA. Germ layer-specific markers were detected by PCR and subsequent agarose gel electrophoresis using primers for the neuroepithelial (ectoderm) markers NESTIN (NES) and NEUROPILIN 2 (NETO2), the endoderm markers SOX17 and α-FETOPROTEIN (AFP) and the mesoderm markers BRACHYURY (T), MATRILIN1 (MANTN1), KDR, and MYF5. GAPDH was used as a loading control. (C) Directed differentiation of human pluripotent cells into early endodermal, ectodermal and mesodermal cells as described (Materials and Methods). Expression of the early lineage markers OTX2 (ectoderm), BRACHYURY (T, mesoderm) and SOX17 (endoderm) was detected by immunofluorescence microscopy using fluorescein-conjugated secondary antibodies. Cell nuclei were detected using DAPI (blue).
