Figure 9. Model for pilus assembly and termination in Enterococcus faecalis.
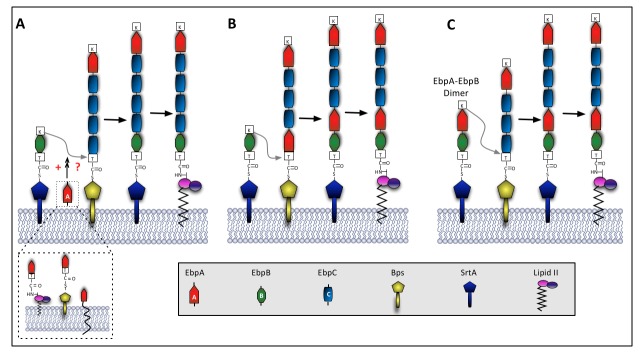
Ebp pilin precursors (EbpA, EbpB and EbpC) are translocated across the membrane by the Sec machinery. This is followed by a sortase-mediated reaction in which membrane-bound EbpA forms an acyl-enzyme intermediate with Bps; then EbpC is presented to this complex by another Bps molecule resulting in cross-linked subunits. Pilus polymerization continues by serial additions of EbpC subunits. We envision several possibilities for termination of Ebp pilus synthesis. (A) - The presence of cell-attached EbpA (membrane-bound, sortase-bound or anchored to the cell wall peptidoglycan) in some way stimulates the incorporation of EbpB into the pilus fiber, terminating pilus polymerization and promoting cell-wall anchoring. (B) - EbpA could be incorporated into the growing chain by Bps, which then signals addition of EbpB as the last subunit and transfer of the polymer to SrtA, which then covalently anchors the nascent pilus to the cell wall peptidoglycan. (C) -Alternatively, EbpA and EbpB could form dimers which are then incorporated into the pilus fiber, followed by transfer of the polymer to SrtA with EbpB as the last subunit of the fiber, which is then covalently anchor to the cell wall. Another possibility (not shown) is that the relative abundance of EbpA influences pilus elongation, perhaps by stabilizing the EbpB; then when EbpA is not present, as in the ebpA deletion mutant, EbpB becomes limited and pilus termination is less frequent.
