Abstract
Background
Iodide uptake at the basolateral membrane and iodide efflux at the apical membrane of thyrocytes, essential steps in the biosynthesis of thyroid hormone, are stimulated by thyroid stimulating hormone (TSH). Pendrin (SLC26A4) is inserted into the apical membrane of thyrocytes and thought to be involved in mediating iodide efflux.
Methods
We determined the effects of carboxy-terminal mutations of pendrin on the cellular localization and the ability to transport iodide.
Results
After exposure to forskolin, the membrane abundance of wild type pendrin and iodide efflux increase. Truncation mutants lead to complete intracellular retention. Elimination of the distal part of the sulfate transporter and antisigma factor antagonist (STAS) domain with retention of the putative protein kinase A (PKA) phosphorylation site (RKDT 714-717) results in residual membrane insertion and a partial loss of function. Deletion of the PKA site results in decreased basal function and membrane insertion and abolishes the response to forskolin.
Conclusion
Pendrin membrane abundance and its ability to mediate iodide efflux increase after activation of the PKA pathway. Elimination of the PKA site abolishes the response to forskolin but partial basal function and membrane insertion are maintained.
Key Words: Pendrin, SLC26A4, Mutation, Iodide transport, Thyroid hormone synthesis
Introduction
Pendred syndrome is an autosomal recessive disorder characterized by sensorineural deafness, goiter and a partial defect in iodide organification [1, 2, 3]. Pendred syndrome is caused by biallelic mutations in the SLC26A4 gene, which encodes the multifunctional anion exchanger pendrin [1]. Currently, more than 170 mutations in the SLC26A4 gene have been documented in patients with Pendred syndrome (http://www.healthcare.uiowa.edu/ laboratories/pendredandbor/slcMutations.htm). The majority of the studied mutations have been shown to lead to abnormal plasma membrane targeting and retention in the endoplasmic reticulum [4, 5, 6]. As a result, mutant proteins lose their ability to transport iodide [6, 7, 8, 9]. A subset of mutations retain membrane insertion but loose the anion exchange function [6, 10, 11, 12].
Pendrin belongs to the Solute Carrier Family 26A (SLC26A), which includes several anion transporters, as well as the motor protein prestin that is expressed in outer hair cells [13, 14, 15]. Like other members of the SLC26A family, pendrin contains a so-called STAS (sulfate transporter and antisigma factor antagonist) domain in its carboxy-terminus (Fig. 1) [16]. This domain shares similarity with the bacterial anti-sigma factor antagonists [16]. Recently, the structure of the STAS domain from the SulP/SLC26 putative anion transporter Rv1739 from Mycobacterium tuberculosis has been solved [17]. Although, the exact role of the STAS domain has not been elucidated, it may play a role in nucleotide binding and/or interactions with other proteins [16, 17, 18, 19, 20].
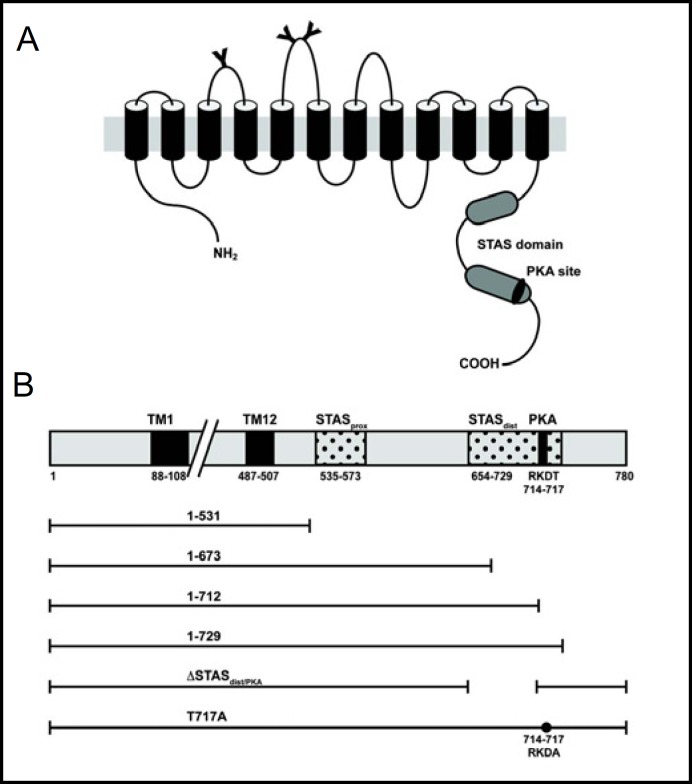
Fig. 1.
Secondary structure of pendrin and schematic presentation of the carboxy-terminal truncation mutants. (A) Current model of the secondary structure of the pendrin protein. Y = putative N-glycosylation sites, STAS = sulfate transporter and antisigma factor antagonist domain. (B) Schematic presentation of the carboxy-terminal truncation mutants of pendrin. TM1, transmembrane helix 1; TM12, transmembrane helix 12; PKA, putative phosphokinase A phosphorylation site. TM numbering based on Uniprot (http://www.uniprot.org/uniprot/O43511), STAS domain based on Aravind et al. [16].
Functional studies in heterologous expression systems demonstrated that pendrin can mediate transport of chloride, bicarbonate, iodide, formate, and thiocyanate [9, 21, 22, 23, 24]. Abundant expression of pendrin can be found in the thyroid, the kidney, and the inner ear [23, 25, 26]. In the thyroid, pendrin localizes to the apical membrane of thyroid follicular cells [25], where it is thought to mediate, at least in part, iodide efflux into the follicular lumen [27].
Both iodide uptake at the basolateral membrane and iodide efflux at the apical membrane of thyrocytes are stimulated by thyroid stimulating hormone (TSH) [28, 29, 30, 31, 32, 33]. However, the exact mechanisms leading to iodide efflux remain poorly characterized. The translocation of pendrin from cytosolic compartments to the membrane is partially mediated by TSH via the PKA pathway [34]. The intracellular carboxy-terminus contains one putative protein kinase A (PKA) site, which may be of importance in the TSH-dependent insertion of pendrin in the apical membrane and, hence, the regulation of iodide efflux [34].
In this study, we characterized the cellular localization and the ability to transport iodide of 1) three truncation mutations in the carboxy-terminus of pendrin, which included the STAS domain and the putative PKA phosphorylation site; 2) a deletion mutation lacking the distal part of the STAS domain but retaining the PKA site; and 3) a mutant with a modified PKA site.
Materials and Methods
Cell culture
TSA-201 cells, a clone of human embryonic kidney 293 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS), penicillin (100 units/ml), and streptomycin (100 µg/ml). Rat kangaroo kidney epithelium (PtK2) cells were cultured in MEM (Invitrogen Gibco) supplemented with 10% FBS, penicillin (100 units/ml), streptomycin (100 µg/ml), and 0.1 mM nonessential amino acids. Calf pulmonary artery endothelial (CPAE) cells were cultured in DMEM containing 20% FBS, penicillin (100 units/ml), and streptomycin (100 µg/ml). All cell lines were maintained in an atmosphere of 5% CO2 at 37° C. Forskolin (FSK) was purchased from Sigma Chemicals (St. Louis, MO). 35 mm glass-bottom culture dishes with coverslip thickness of 0.16 to 0.19 mm were from MatTek (MatTek Corporation, Ashland, MA).
Construction of mutants
Carboxy-terminal truncation mutants of pendrin were generated by PCR using a pEGFPN1 plasmid containing the wild type PDS cDNA as template. The following five mutants were constructed (Fig. 1): 1) del531 mutant (residues 1-531) lacking the terminal 249 amino acids with the entire STAS domain; 2) del673 mutant (residues 1-673) lacking the terminal 107 amino acids; 3) del712 mutant (residues 1-712) lacking the terminal 68 amino acids including the putative PKA phosphorylation site; 4) del729 mutant (residues 1-729) lacking the terminal 51 amino acids of the full-length protein while retaining the STAS domain, and 5) ASTASdist/PKA lacking the distal STAS domain (residues 654-712) but retaining the PKA site. After PCR amplification with primers containing an appropriate linker, the amplified fragments were subcloned in-frame and without stop codon into the BamHI and HindIII sites of the pEGFPN1 vector. All final constructs were confirmed by sequencing. A EGFP-tagged pendrin mutant containing a modified putative PKA phosphorylation site, in which threonine was substituted for alanine at position 717 (PDS T717A), was a gift from Dr. Liuska Pesce.
Transfection
For iodide assays, a pCMX plasmid containing the NIS cDNA (50 ng/well), and pEGFPN1 plasmids carrying the wild type pendrin (PDS) cDNA or truncated PDS mutants fused to a carboxy-terminal EGFP (10 ng/well) were transfected using the calcium phosphate method. The empty pCMX and pEGFPN1 vectors were used as negative controls. In contransfection experiments of NIS and PDS constructs, the total amount of DNA was kept constant by adding empty vector.
Transfection of rat kangaroo kidney epithelium (PtK2) and calf pulmonary artery endothelial (CPAE) cells was performed by electroporation. Cells were trypsinized and pelleted by centrifugation. Pelleted cells were resuspended in 600 µl of OptiMEM medium (Invitrogen). 200 µl of the resuspended cells were mixed with 5 µg DNA containing 6.5 µg sheared salmon sperm DNA and electroporated in 4 mm cuvettes using a Bio-Rad Laboratories Gene Pulser set to 190 Ohms, 950 µFD.
Iodide uptake
Iodide uptake assays were performed 48 hours following transfection of TSA cells grown to 80% confluency in 12-well tissue culture plates. Cells were washed once with 1x PBS and incubated with Hanks’ balanced salt solution containing 10 mM HEPES (pH 7.4), 1 mM methimazole, 1 mM DTT, and 10-5 M cold NaI labeled with Na125I (20 mCi/mmol) for 30 minutes at 37° C. During the exposure to iodide uptake solution, TSA cells were incubated in the absence or in the presence of 50 µM forskolin. The radiolabeled iodide solution was aspirated and cells were permeabilized using 1% Triton-X in PBS. The intracellular iodide content was determined by quantifying the amount of radiolabeled iodide in the cell lysates using a gamma counter.
Live cell imaging
PtK2 cells transfected with EGFP-tagged wild type pendrin or the respective mutants were imaged using an Olympus DSU spinning disk confocal fitted on an Olympus IX-81 microscope enclosed in a 37°C heated CO2 chamber in the Northwestern University Cell Imaging Facility. Cells were examined under a 60x oil-immersion objective. Images were acquired at a 100 ms exposure every 5 minutes. After one hour, forskolin (50 µM) was added to the cells and images were collected for another hour. In order to image cells in the absence of CO2, the cell medium was mixed with equal volumes of Leibovitz L-15 medium without Phenol Red (Invitrogen Gibco) supplemented with 10% FBS. For long-term live imaging, the focal plane was actively maintained by the zero drift compensation mechanism of the Olympus IX-81 microscope.
Immunostaining analysis
Calf pulmonary artery endothelial (CPAE) cells transiently transfected with EGFP-tagged wild type and mutants were grown on coverslips. Twenty-four hours following transfection, cells were fixed in 3.7% paraformaldehyde for 5 minutes at room temperature, permeabilized with 0.2% Triton X-100 in PBS for 5 minutes at room temperature, blocked with 0.1% BSA for 15 minutes, and incubated with a chicken polyclonal anti-calreticulin antibody (1:200 dilution, Abcam) for 30 minutes. After three washes with PBS, cells were incubated with goat anti-chicken Alexa568 (1:200 dilution, Molecular Probes, Invitrogen) for 30 minutes, counterstained with DAPI and mounted. Immunolocalization images were taken with a Zeiss LSM 510 Meta confocal laser scanning microscope.
Sequence alignment
The multiple sequence alignment of the carboxy-terminal STAS domains of 10 SLC26A transporters was obtained using T-coffee (http://www.tcoffee.org/homepage.html). The location of conserved residues was shaded using the Boxshade program (http://www.ch.embnet.org/software/BOX_form.html). The STAS domain was designated based on the sequence comparisons by Aravind et al. [16].
Statistical analysis
All analyses were performed in at least three independent experiments. Data are reported as means ± SEM. Statistical analysis was performed by one-way ANOVA, using Tukey's correction using Prism (GraphPad Software, La Jolla, CA). Results were considered significant when p < 0.05.
Results
Based on prediction programs such as Uniprot (http://www.uniprot.org/uniprot/O43511), pendrin is predicted to have 12 transmembrane domains with both the amino-and carboxy-termini located inside the cytosol (Fig. 1A) [25, 27]. However, alternative models have also been proposed [1, 9]. In order to assess the role of the cytoplasmic carboxy-terminus tail of pendrin, we studied four mutants with truncations of the carboxy-terminus (Fig. 1B). In order to determine whether the PKA site and the distal part of the STAS domain play an important role in pendrin's insertion into the plasma membrane, we have analyzed a pendrin mutant containing a modified putative PKA phosphorylation site, as well as a mutant lacking the distal part of the STAS domain but maintaining an intact PKA site and carboxy-terminus. Each mutant was analyzed in terms of functionality, specifically the ability to mediate iodide efflux from transfected cells, and its subcellular localization using live imaging and indirect immunofluorescence.
Functional analysis of iodide transport
Functional studies have shown that pendrin is able to mediate iodide efflux at the apical membrane of polarized and from non-polarized heterologous cells [27]. In order to assess the effect of the mutations on the ability of pendrin to mediate iodide efflux, we cotransfected nonpolarized TSA cells, lacking endogenous pendrin expression, with the sodium iodide transporter NIS to allow cells to accumulate iodide, and determined radiolabeled iodide content in the absence and in the presence of stimulation with forskolin. All assays were performed without treating the cells with perchlorate, a competitive inhibitor of NIS, during the treatment with forskolin.
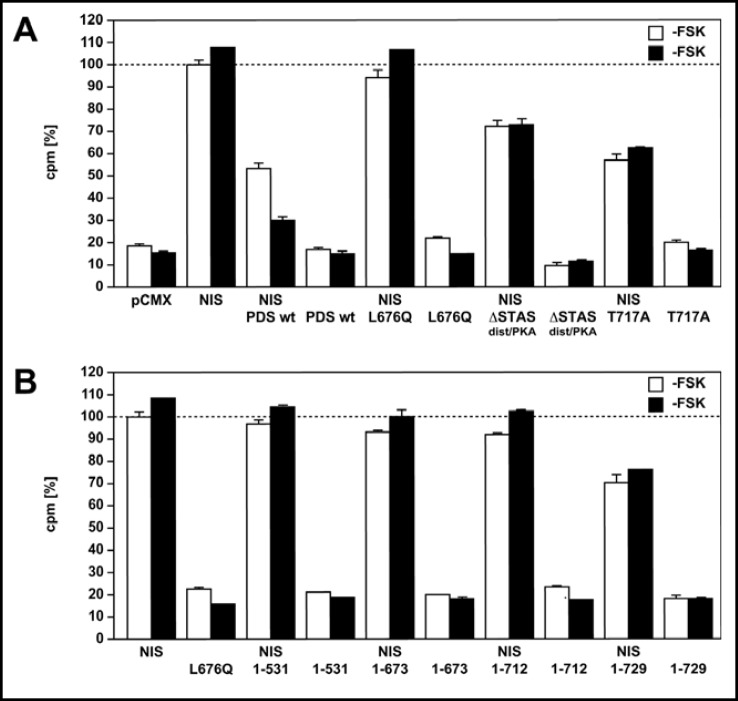
TSA cells transiently expressing NIS alone demonstrate an 82% increase (p <0.001) in iodide uptake in the absence of forskolin, compared with control cells transfected with the empty vector (Fig. 2A). In the presence of forskolin, cells expressing NIS accumulate a higher amount of intracellular iodide of 90% (-FSK vs. +FSK p <0.05), an increase thought to result from increased NIS activity (Fig. 2A) [35]. Cells transfected with pendrin alone did not show an increase in iodide uptake in comparison to control cells. In cells cotransfected with NIS and pendrin in the absence of forskolin, the intracellular iodide amount is reduced by 47% (p <0.001), and incubation with forskolin leads to a further decrease by 71% (p <0.05) in iodide content (-FSK vs. +FSK p <0.001, Fig. 2A). Cotransfection of cells with NIS and the previously characterized pendrin loss-of-function mutant L676Q, a mutant found in a patient with Pendred syndrome [27], does not result in a decrease in intracellular iodide, and served as a positive control (Fig. 2A). Cells coexpressing NIS and the pendrin mutant with the eliminated distal STAS domain but an intact PKA domain (ASTASdist/PKA) show a 28% decrease (p <0.001) in intracellular iodide compared to cells expressing NIS in the absence and in the presence of forskolin, indicating that the mutant retains a reduced but partially retained ability to mediate iodide efflux (Fig. 2A). Cotransfection of NIS and a pendrin mutant containing the modified PKA phosphorylation site (PDS T717A) led to a 43% and 38% decrease in intracellular iodide in the absence and in the presence of forskolin (-FSK vs. +FSK p >0.05), respecti-vely, showing that the mutant is partially functional but indicating that the response to forskolin is mitigated (Fig. 2A). Coexpression of the three truncation mutants (PDS 1-531 + NIS; PDS 1-673 + NIS; PDS 1-712 + NIS) does not cause a decrease (p >0.05) in intracellular iodide accumulation in the absence and in the presence of forskolin, compared to cells transfected with NIS only, indicating that these mutants lose their ability to transport iodide (Fig. 2B). Cotransfection of NIS and the shortest truncation mutant 1-729 containing the putative PKA phosphorylation site, results in a 30% and 25% decrease in intracellular iodide accumulation in the absence and in the presence of forskolin, respectively (-FSK vs. +FSK p>0.05), but the reduction is significantly lower compared to the wild type (1-729 versus NIS/PDS: p<0.001; 1-729+FSK versus NIS/PDS+FSK: p<0.001). This suggests that the mutant retains a partial ability to mediate iodide efflux, but that it is unable to respond to stimulation by the PKA pathway (Fig. 2B).
Fig. 2.
Intracellular iodide content of TSA cells expressing wild type and mutant pendrin proteins. A) Function of wild type and mutant pendrin proteins was determined by assessing intracellular iodide content using radiolabeled iodide. Cells were analyzed without forskolin treatment (white bars) or after exposure to forskolin (black bars); the forskolin treatment was performed without blocking NI S with perchlorate. TSA cells transiently expressing NIS alone show a 82% and 90% increase (p <0.05) in intracellular iodide content in the absence and in the presence of forskolin respectively, compared with control cells transfected with an empty vector (-FSK vs. +FSK p <0.05). Cotransfection of NIS and PDS leads to a significant decrease in iodide content in the absence and in the presence of forskolin (47% and 71%, respectively, p <0.001). Cotransfection of the previously characterized loss-of-function mutant L676Q and NIS does not result in a decrease in intracellular iodide content under both conditions, and serves as a positive control. Cells coexpressing NIS and a pendrin mutant with deletion of the distal STAS domain and an intact PKA site (ASTASdist/PKA) show a 28% decrease (p <0.001) in intracellular iodide content compared to cells expressing NIS in the absence and in the presence of forskolin, indicating that the mutant retains a partial ability to mediate iodide efflux but that it does not respond to forskolin. Contransfection of NIS and the PDS T717A mutant results in a 43% and 38% decrease in intracellular iodide content in the absence and in the presence of forskolin, respectively (-FSK vs. +FSK p >0.05), compared to cells expressing NIS only, suggesting that the mutant is partially functional but that it has a mitigated response to forskolin; the slight increase in intracellular iodide may be mediated by stimulation of NIS, which was not blocked by perchlorate. B) For comparison to cells transfected with NIS, the bars indicating the iodide accumulation in cells transfected with NIS are reproduced from panel 2A. Cells coexpressing the three truncation mutants (PDS 1-531+NIS; PDS 1-673+NIS; PDS 1-712+NIS) do not show a decrease (p >0.05) in intracellular iodide amount compared to cells expressing NIS only, indicating that these mutants lose their ability to mediate iodide transport. Cotransfection of NIS and the shortest truncation mutant 1-729 containing the putative PKA phosphorylation site, results in a 30% and 25% decrease in intracellular iodide accumulation in the absence and in the presence of forskolin, respectively (-FSK vs. +FSK p >0.05), but the reduction is significantly lower compared to the wild type (1-729 versus NIS/PDS: p<0.001; 1-729+FSK versus NIS/PDS+FSK: p<0.001). This suggests that the mutant retains a partial ability to mediate iodide efflux, but that it is unable to respond to stimulation by the PKA pathway. Data shown here are representative of at least three independent experiments. Values are the means of triplicates ± SEM.
Live imaging
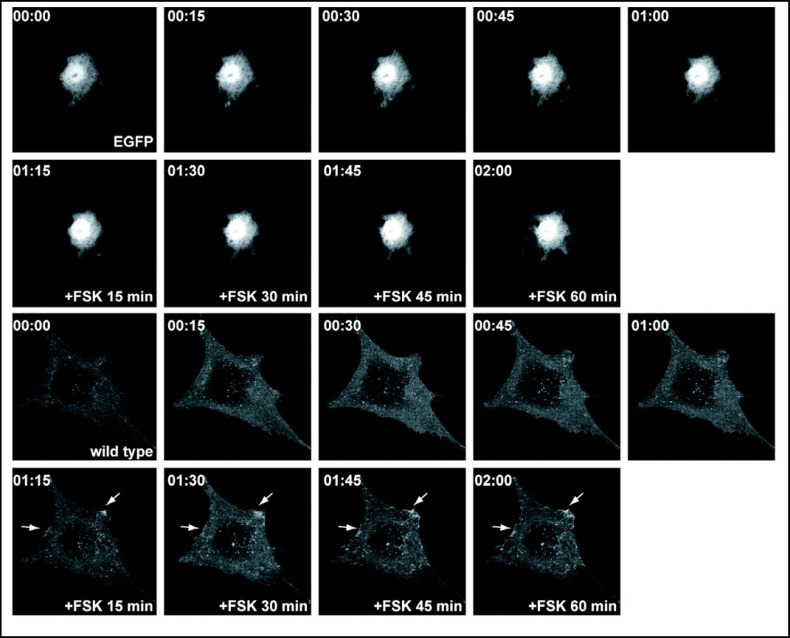
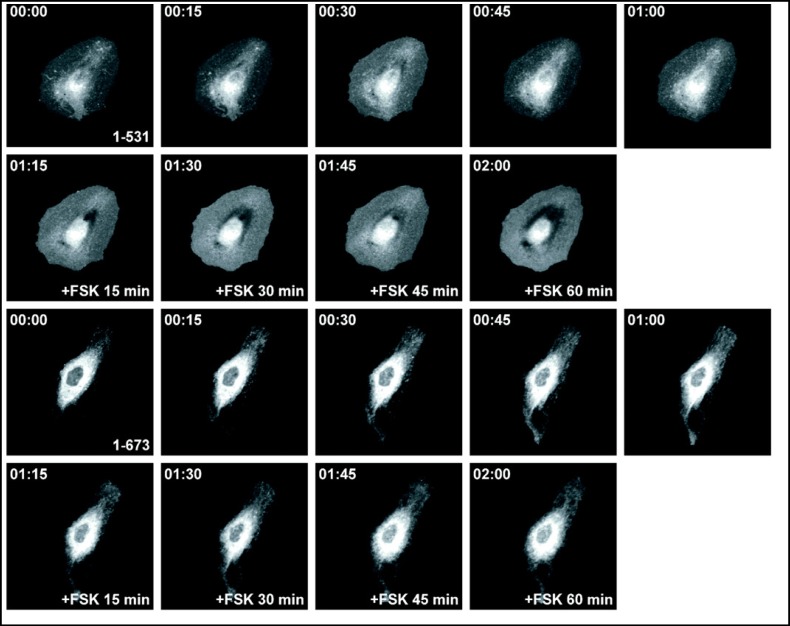
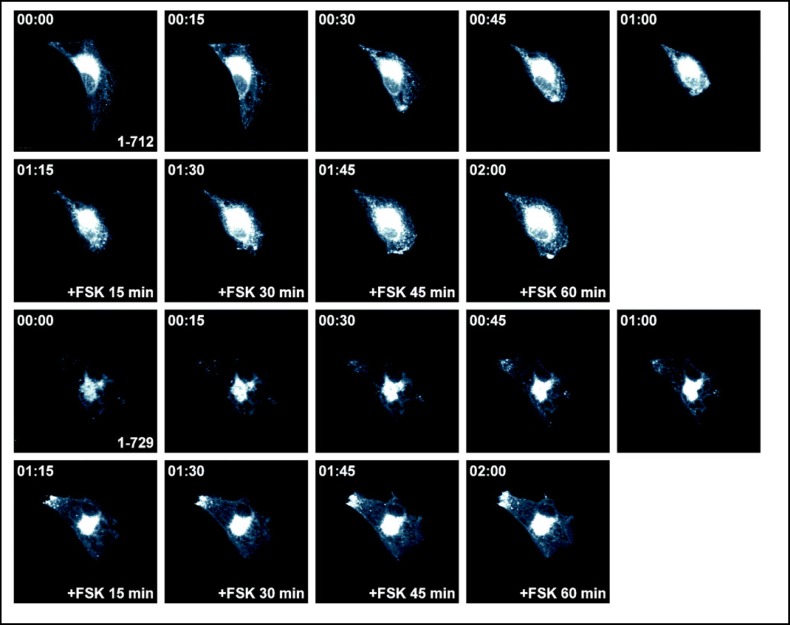
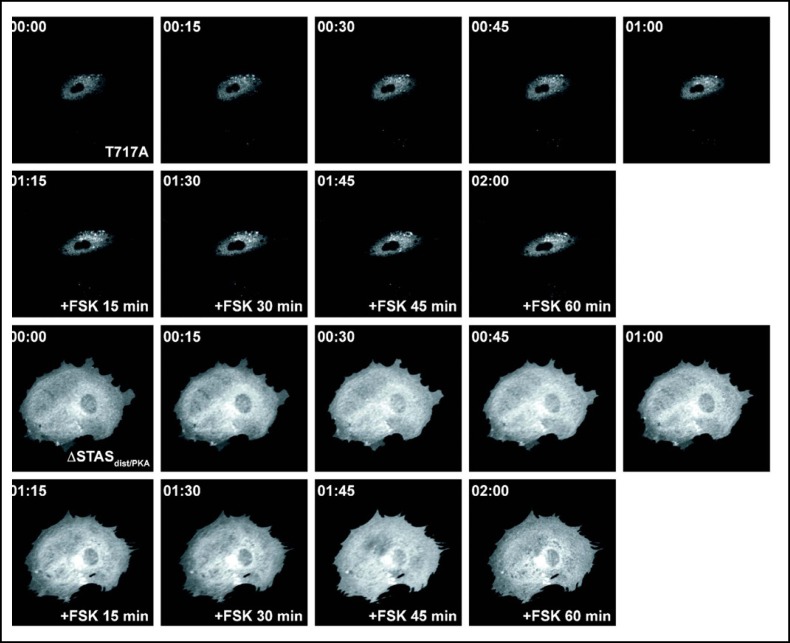
To assess the effects of truncations on plasma membrane targeting, we expressed wild type and mutant EGFP-tagged pendrin in PtK2 cells and analyzed the localization using time-lapse imaging of living cells. In the absence of forskolin, the majority of the EGFP-tagged wild type pendrin is located in intracellular compartments (Fig. 3). A similar pattern was observed in cells expressing truncation mutants in the absence of forskolin. Incubation with forskolin increases the membrane abundance of wild type pendrin with a pattern that is suggestive for the formation of protein clusters within the lamellipodial protrusions of the cell (Fig. 3). In contrast, the majority of the EGFP-tagged truncation mutants with a deleted or shortened STAS domain (PDS 1-531, PDS 1-673) (Fig. 4), and the putative PKA phosphorylation site (PDS 1-712) fail to reach the plasma membrane and are retained inside the cytosol after incubation with forskolin for one hour (Fig. 5). A significant amount of the EGFP-tagged truncation mutant containing both the STAS domain and the putative PKA phosphorylation site (PDS 1-729) is retained in the cytoplasm (Fig. 5). The majority of the pendrin mutant lacking the distal STAS domain with an intact PKA site (ASTASdist/PKA), as well as the mutant containing a modified PKA phosphorylation site are predominantly located in the cytoplasm (Fig. 6) although the functional studies are indicating a partial residual function (Fig. 2). The results reported here are representative of at least five independent experiments.
Fig. 3.
Subcellular distribution of the EGFP-tagged wild type pendrin in living PtK2 cells. PtK2 cells transiently expressing EGFP-tagged wild type pendrin were imaged using time-lapse confocal microscopy. Cells transfected with pEGFP empty plasmid served as control. In the absence of forskolin, the majority of the wild type pendrin is retained in intracellular compartments. Within 15 minutes after adding forskolin, the membrane abundance increases with a pattern that is suggestive for the formation of protein clusters within the lamellipodial protrusions of the cell (indicated by arrows). This pattern is observed for one hour following treatment with forskolin. These results suggest that plasma membrane targeting of pendrin is mediated, in part, by the PKA pathway.
Fig. 4.
Subcellular distribution of the EGFP-tagged pendrin truncation mutants 1-531 and 1-673 in living PtK2 cells. PtK2 cells transiently expressing EGFP-tagged pendrin truncation mutants were imaged using time-lapse confocal microscopy. In the absence of forskolin, the majority of the truncation mutants 1-531 and 1-673 remain in intracellular compartments. The subcellular distribution of the truncation mutants remains unchanged following treatment with forskolin. These results indicate that these mutants fail to reach the plasma membrane following activation of the PKA pathway.
Fig. 5.
Subcellular distribution of the EGFP-tagged pendrin truncation mutants 1-712 and 1-729 in living PtK2 cells. PtK2 cells transiently expressing EGFP-tagged pendrin truncation mutants were imaged using time-lapse confocal microscopy. In the absence of forskolin, the majority of the truncation mutants 1-712 and 1-729 remain in intracellular compartments. In the presence of forskolin, there is no visible change in membrane insertion. In the functional assay (Fig. 2B), 1-729 retains a partial basal function but does not respond to forskolin.
Fig. 6.
Subcellular distribution of the EGFP-tagged pendrin mutant containing a modified putative PKA phosphorylation site (PDS T717A) and the mutant lacking the distal STAS domain but containing an intact PKA site and carboxy-terminus (ASTASdist/PKA) in living PtK2 cells. PtK2 cells transiently expressing the EGFP-tagged pendrin mutants were imaged using time-lapse confocal microscopy. In the absence of forskolin, the majority of the mutants PDS T717A and the ASTASdist/PKA are located in intracellular compartments. In the functional assay both mutants have a partially retained function, although significantly reduced compared to the wild type (Fig. 2A). In the presence of forskolin, there is no visible change in membrane insertion, which
Subcellular distribution of pendrin and mutant proteins
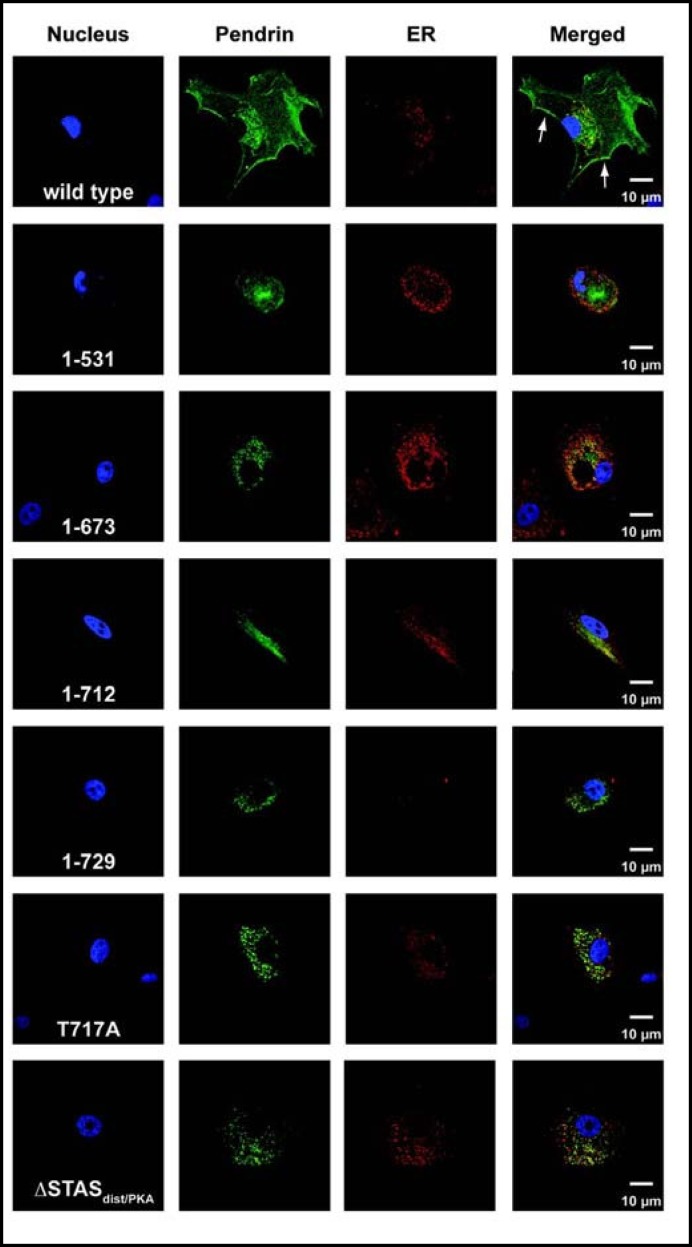
To determine the intracellular distribution of mutant proteins, we expressed EGFP-tagged wild type and mutant pendrin in CPAE cells and examined expression patterns of the proteins by colocalization of pendrin with calreticulin, which served as an endoplasmic reticulum (ER) marker (Fig. 7). Twenty-four hours after transfection, EGFP-tagged wild type pendrin showed significant membrane localization with a small fraction of the protein present in the ER (Fig. 7A). None of the pendrin mutants showed significant insertion into the cell membrane and demonstrated significant ER accumulation (Fig. 7B-G). Using calreticulin, an ER specific marker, we confirmed colocalization of the mutant proteins with the ER.
Fig. 7.
Cellular localization of wild type pendrin and mutants 1-531, 1-673, 1-712, 1-729, T717A, and ASTASdist/PKA. Twenty-four hours after transfection with the EGFP-tagged wild type and mutant pendrin proteins, CPAE cells were stained with an anti-calreticulin antibody. A significant amount of wild type pendrin showed ER localization whereas a small portion of the protein was present at the plasma membrane (shown by arrowheads). All mutant proteins showed predominant ER localization, as demonstrated by colocalization of the proteins with calreticulin.
Discussion
In this study, we have analyzed the consequences of carboxy-terminal pendrin mutants on the insertion of pendrin into the plasma membrane and the ability to transport iodide. Functional analysis of iodide uptake in TSA cells transiently expressing NIS alone demonstrate a higher increase in intracellular iodide accumulation in the presence of forskolin compared to cells expressing NIS in the absence of forskolin (82% versus 90% increase compared to untransfected and untreated cells, Fig. 2A). Cotransfection of NIS and pendrin leads to a 47% decrease in iodide content in the absence of forskolin, and even a larger decrease (71%) in the presence of forskolin (Fig. 2A). These results are consistent with the established evidence that TSH, the main regulator of thyroid cell function and growth, increases iodide uptake at the basolateral membrane of thyroid cells and iodide efflux at the apical membrane into the follicular lumen [28, 30, 31, 32, 33]. It is known that TSH up-regulates iodide uptake via the cAMP pathway by modulating NIS expression via transcriptional and post-transcriptional mechanisms [35, 36, 37]. Moreover, a recent study by Pesce et al. has shown that TSH stimulates the insertion of pendrin into the plasma membrane as well as iodide efflux in thyroid cells following the inhibition of iodide uptake by sodium perchlorate, via post-transcriptional mechanisms mediated by the PKA-dependent pathway [34]. The results of our functional analysis, although obtained in the absence of incubation with sodium perchlorate during the treatment with forskolin, are consistent with the latter study.
The results of our study show that the membrane abundance of pendrin appears to increase and the protein seems to form protein clusters within lamellipodial extensions of the cell within fifteen minutes after addition of forskolin (Fig. 3). Functionally, this is paralleled by an increase in iodide efflux (Fig. 2A). In contrast to wild type pendrin, EGFP-tagged truncation mutants lacking an intact STAS domain and the putative PKA phosphorylation site fail to show an increase in membrane abundance in response to forskolin (Fig. 4, 5, 6).
Like all members of the SLC26A4 family, pendrin carries a STAS domain in its carboxy-terminus. While the role of the STAS domain needs further characterization, it has been suggested that it is involved in nucleotide binding and/or interactions with other proteins [16, 17, 19, 20]. The STAS domain of pendrin is thought to encompass amino acids 535 to 573 and residues 654 to 729 [16] (Fig. 8). Functional studies of mutations reported in patients with Pendred syndrome and familial enlarged aqueduct (EVA) indicate that the majority of the mutations found in the STAS domain lead to abnormal plasma membrane targeting and retention in intracellular compartments [1, 4, 6, 7, 8, 27]. These mutations display a reduced or a complete loss of iodide efflux [1, 4, 6, 7, 8, 27].
Fig. 8.
Alignment of the carboxy-terminal regions including the STAS domains of human SLC26A transporters. The carboxy-terminal STAS domains of 10 human SLC26A transporters were aligned using T-coffee and then shaded using the Boxshade program. The predicted domain boundaries of the SLC26A4 STAS domain according to Aravind et al. (residues 535 to 573 and 654 to 729) [16] are boxed. The asterisk indicates the position of known mutations causing Pendred syndrome and EVA, found in the SLC26A4 STAS domain.
Some of these mutations include point mutations in the conserved residues of the STAS domain (Fig. 8). A mutant lacking the distal STAS domain but retaining the PKA site, ASTASdist/PKA, and maintaining an intact carboxy-terminal tail, is predominantly localized in the cytoplasm (Fig. 6); functionally it retains partial basal function, but does no longer respond to forskolin (Fig. 2A).
Elimination of the PKA phosphorylation site (PDS T717A mutant), as well as the deletion of carboxy-terminal sequences downstream of the putative PKA phosphorylation site (PDS 1-729 mutant) leads to a residual membrane insertion and a partial to mediate transport of iodide but abolished the response to forskolin (Fig. 2, 5). These findings suggest that pendrin can translocate partially to the plasma membrane without a functional PKA site but that a rapid insertion in response to forskolin, as observed with the wild type, is no longer possible. Whether the 1-712 truncation mutant can no longer be phosphorylated, will need to be addressed in additional studies. In addition to the presence of the putative PKA phosphorylation site at position 714-717, pendrin also contains several potential phosphorylation sites for protein kinase C and Muscella et al. have demonstrated translocation of pendrin from the cytosol to the plasma membrane after activation of the PKC pathway following exposure of cultured rat thyroid PCCl3 cells to insulin for 10, 20 and 40 minutes [38]. These findings contrast with other results suggesting that plasma membrane targeting of pendrin is regulated via the PKA-dependent pathway [34]. In particular, in PCCl3 cells pendrin abundance at the plasma membrane increased following a 5 minute incubation with TSH as demonstrated by immunoblotting and immunofluorescence [34]. The abundance of pendrin at the plasma membrane decreased following incubation with the PKA inhibitor H-89, whereas the PKC inhibitor bisindolylmaleimide failed to block the increase in translocation to the membrane [34]. Based on the currently available data it is possible that the PKA-dependent pathway exerts a short-term effect, whereas the PKC-dependent pathway results in a delayed effect on cytosol-to-membrane translocation of pendrin. Our results show that the PKA mutant (PDS T717A) remains partially active suggesting that phosphorylation of the putative PKA site located at position 714-717 does not serve as an absolute prerequisite for targeting of pendrin to the plasma membrane. It is possible that other post-translational modifications, such as glycosylation may have to occur together with phosphorylation in order for translocation to the plasma membrane to take place. Human pendrin is known to contain three potential N-glycosylation sites; their potential role in the cellular localization and function of pendrin has, however, not been determined. In murine pendrin, glycosylation does not seem to be of importance for membrane insertion and basal activity in terms of chloride/bicarbonate exchange, but disruption of the glycosylation sites abrogates the sensitivity of mouse pendrin to extracellular pH [39].
Limitations of this study consist in the lack of quantitative morphological or biochemical assays assessing the protein amount in the membrane. Moreover, additional deletions mutants of the STAS domain are needed to further characterize its functional role.
In conclusion, truncation deletions encompassing the STAS domain lead to a complete loss of function and retention of the mutants in intracellular compartments. Partial deletion of the distal part of the STAS domain leads to a reduced plasma membrane insertion and consequently in a decreased ability to mediate iodide efflux. Lastly, deletion of the PKA site, as well as elimination of the carboxy-terminal sequences downstream of this site, lead to partial membrane insertion and a residual iodide transport activity, suggesting that partial membrane insertion is not dependent on the presence of this site.
Acknowledgements
This work has been supported, in part, by Grant 1R01DK63024-01 from NIDDK, National Institutes of Health (to PK), and a gift from Mr. David Wiener (to PK).
References
- 1.Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, Green ED. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS) Nat Genet. 1997;17:411–422. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- 2.Bizhanova A, Kopp P. Genetics and phenomics of Pendred syndrome. Mol Cell Endocrinol. 2010;322:83–90. doi: 10.1016/j.mce.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Kopp P, Pesce L, Solis SJ. Pendred syndrome and iodide transport in the thyroid. Trends Endocrinol Metab. 2008;19:260–268. doi: 10.1016/j.tem.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Yoon JS, Park HJ, Yoo SY, Namkung W, Jo MJ, Koo SK, Park HY, Lee WS, Kim KH, Lee MG. Heterogeneity in the processing defect of SLC26A4 mutants. J Med Genet. 2008;45:411–419. doi: 10.1136/jmg.2007.054635. [DOI] [PubMed] [Google Scholar]
- 5.Rotman-Pikielny P, Hirschberg K, Maruvada P, Suzuki K, Royaux IE, Green ED, Kohn LD, Lippincott-Schwartz J, Yen PM. Retention of pendrin in the endoplasmic reticulum is a major mechanism for Pendred syndrome. Hum Mol Genet. 2002;11:2625–2633. doi: 10.1093/hmg/11.21.2625. [DOI] [PubMed] [Google Scholar]
- 6.Taylor JP, Metcalfe RA, Watson PF, Weetman AP, Trembath RC. Mutations of the PDS gene, encoding pendrin, are associated with protein mislocalization and loss of iodide efflux: implications for thyroid dysfunction in Pendred syndrome. J Clin Endocrinol Metab. 2002;87:1778–1784. doi: 10.1210/jcem.87.4.8435. [DOI] [PubMed] [Google Scholar]
- 7.Pera A, Dossena S, Rodighiero S, Gandia M, Botta G, Meyer G, Moreno F, Nofziger C, Hernandez-Chico C, Paulmichl M. Functional assessment of allelic variants in the SLC26A4 gene involved in Pendred syndrome and nonsyndromic EVA. Proc Natl Acad Sci USA. 2008;105:18608–18613. doi: 10.1073/pnas.0805831105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott DA, Wang R, Kreman TM, Andrews M, McDonald JM, Bishop JR, Smith RJ, Karniski LP, Sheffield VC. Functional differences of the PDS gene product are associated with phenotypic variation in patients with Pendred syndrome and non-syndromic hearing loss (DFNB4) Hum Mol Genet. 2000;9:1709–1715. doi: 10.1093/hmg/9.11.1709. [DOI] [PubMed] [Google Scholar]
- 9.Dossena S, Rodighiero S, Vezzoli V, Nofziger C, Salvioni E, Boccazzi M, Grabmayer E, Botta G, Meyer G, Fugazzola L, Beck-Peccoz P, Paulmichl M. Functional characterization of wild-type and mutated pendrin (SLC26A4), the anion transporter involved in Pendred syndrome. J Mol Endocrinol. 2009;43:93–103. doi: 10.1677/JME-08-0175. [DOI] [PubMed] [Google Scholar]
- 10.Dossena S, Vezzoli V, Cerutti N, Bazzini C, Tosco M, Sironi C, Rodighiero S, Meyer G, Fascio U, Furst J, Ritter M, Fugazzola L, Persani L, Zorowka P, Storelli C, Beck-Peccoz P, Botta G, Paulmichl M. Functional characterization of wild-type and a mutated form of SLC26A4 identified in a patient with Pendred syndrome. Cell Physiol Biochem. 2006;17:245–256. doi: 10.1159/000094137. [DOI] [PubMed] [Google Scholar]
- 11.Choi BY, Stewart AK, Madeo AC, Pryor SP, Lenhard S, Kittles R, Eisenman D, Kim HJ, Niparko J, Thomsen J, Arnos KS, Nance WE, King KA, Zalewski CK, Brewer CC, Shawker T, Reynolds JC, Butman JA, Karniski LP, Alper SL, Griffith AJ. Hypo-functional SLC26A4 variants associated with nonsyndromic hearing loss and enlargement of the vestibular aqueduct: genotype-phenotype correlation or coincidental polymorphisms? Hum Mutat. 2009;30:599–608. doi: 10.1002/humu.20884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai P, Stewart AK, Chebib F, Hsu A, Rozenfeld J, Huang D, Kang D, Lip V, Fang H, Shao H, Liu X, Yu F, Yuan H, Kenna M, Miller DT, Shen Y, Yang W, Zelikovic I, Platt OS, Han D, Alper SL, Wu BL. Distinct and novel SLC26A4/ Pendrin mutations in Chinese and U.S. patients with nonsyndromic hearing loss. Physiol Genomics. 2009;38:281–290. doi: 10.1152/physiolgenomics.00047.2009. [DOI] [PubMed] [Google Scholar]
- 13.Everett LA, Green ED. A family of mammalian anion transporters and their involvement in human genetic diseases. Hum Mol Genet. 1999;8:1883–1891. doi: 10.1093/hmg/8.10.1883. [DOI] [PubMed] [Google Scholar]
- 14.Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]
- 15.Bizhanova A, Kopp P. Minireview: The sodium-iodide symporter NIS and pendrin in iodide homeostasis of the thyroid. Endocrinology. 2009;150:1084–1090. doi: 10.1210/en.2008-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aravind L, Koonin EV. The STAS domain - a link between anion transporters and antisigma-factor antagonists. Curr Biol. 2000;10:R53–55. doi: 10.1016/s0960-9822(00)00335-3. [DOI] [PubMed] [Google Scholar]
- 17.Sharma AK, Ye L, Baer CE, Shanmuga-sundaram K, Alber T, Alper SL, Rigby AC. Solution structure of the guanine nucleotide-binding STAS domain of SLC26-related SulP protein Rv1739c from Mycobacterium tuberculosis. J Biol Chem. 2011;286:8534–8544. doi: 10.1074/jbc.M110.165449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shcheynikov N, Ko SB, Zeng W, Choi JY, Dorwart MR, Thomas PJ, Muallem S. Regulatory interaction between CFTR and the SLC26 transporters. Novartis Found Symp. 2006;273:177–186. discussion 186-192, 261-174. [PubMed] [Google Scholar]
- 19.Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO3- transport in cystic fibrosis. EMBO J. 2002;21:5662–5672. doi: 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol. 2004;6:343–350. doi: 10.1038/ncb1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott DA, Wang R, Kreman TM, Sheffield VC, Karniski LP. The Pendred syndrome gene encodes a chloride-iodide transport protein. Nat Genet. 1999;21:440–443. doi: 10.1038/7783. [DOI] [PubMed] [Google Scholar]
- 22.Everett LA. New insights into the role of pendrin (SLC26A4) in inner ear fluid homeostasis. Novartis Found Symp. 2006;273:213–225. discussion 225-230, 261-214. [PubMed] [Google Scholar]
- 23.Soleimani M, Greeley T, Petrovic S, Wang Z, Amlal H, Kopp P, Burnham CE. Pendrin: an apical Cl-/OH-/HCO3-exchanger in the kidney cortex. Am J Physiol Renal Physiol. 2001;280:F356–364. doi: 10.1152/ajprenal.2001.280.2.F356. [DOI] [PubMed] [Google Scholar]
- 24.Pedemonte N, Caci E, Sondo E, Caputo A, Rhoden K, Pfeffer U, Di Candia M, Bandettini R, Ravazzolo R, Zegarra-Moran O, Galietta LJ. Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: role of pendrin and anion channels. J Immunol. 2007;178:5144–5153. doi: 10.4049/jimmunol.178.8.5144. [DOI] [PubMed] [Google Scholar]
- 25.Royaux IE, Suzuki K, Mori A, Katoh R, Everett LA, Kohn LD, Green ED. Pendrin, the protein encoded by the Pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinology. 2000;141:839–845. doi: 10.1210/endo.141.2.7303. [DOI] [PubMed] [Google Scholar]
- 26.Everett LA, Morsli H, Wu DK, Green ED. Expression pattern of the mouse ortholog of the Pendred's syndrome gene (Pds) suggests a key role for pendrin in the inner ear. Proc Natl Acad Sci USA. 1999;96:9727–9732. doi: 10.1073/pnas.96.17.9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillam MP, Sidhaye AR, Lee EJ, Rutishauser J, Stephan CW, Kopp P. Functional characterization of pendrin in a polarized cell system. Evidence for pendrin-mediated apical iodide efflux. J Biol Chem. 2004;279:13004–13010. doi: 10.1074/jbc.M313648200. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson M, Bjorkman U, Ekholm R, Ericson LE. Iodide transport in primary cultured thyroid follicle cells: evidence of a TSH-regulated channel mediating iodide efflux selectively across the apical domain of the plasma membrane. Eur J Cell Biol. 1990;52:270–281. [PubMed] [Google Scholar]
- 29.Halmi NS, Granner DK, Doughman DJ, Peters BH, Muller G. Biphasic effect of TSH on thyroidal iodide collection in rats. Endocrinology. 1960;67:70–81. doi: 10.1210/endo-67-1-70. [DOI] [PubMed] [Google Scholar]
- 30.Weiss SJ, Philp NJ, Grollman EF. Iodide transport in a continuous line of cultured cells from rat thyroid. Endocrinology. 1984;114:1090–1098. doi: 10.1210/endo-114-4-1090. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson M, Bjorkman U, Ekholm R, Ericson LE. Polarized efflux of iodide in porcine thyrocytes occurs via a cAMPregulated iodide channel in the apical plasma membrane. Acta Endocrinol (Copenh) 1992;126:67–74. doi: 10.1530/acta.0.1260067. [DOI] [PubMed] [Google Scholar]
- 32.Weiss SJ, Philp NJ, Grollman EF. Effect of thyrotropin on iodide efflux in FRTL-5 cells mediated by Ca2+ Endocrinology. 1984;114:1108–1113. doi: 10.1210/endo-114-4-1108. [DOI] [PubMed] [Google Scholar]
- 33.Weiss SJ, Philp NJ, Ambesi-Impiombato FS, Grollman EF. Thyrotropin-stimulated iodide transport mediated by adenosine 3',5'-monophosphate and dependent on protein synthesis. Endocrinology. 1984;114:1099–1107. doi: 10.1210/endo-114-4-1099. [DOI] [PubMed] [Google Scholar]
- 34.Pesce L, Bizhanova A, Caraballo SC, Westphal W, Butti ML, Lomellas A, Kopp P: Thyroid stimulating hormone (TSH) regulates pendrin membrane abundance and enhances iodide efflux in thyroid cells. Edocrinology 2011; in press. [DOI] [PMC free article] [PubMed]
- 35.Dohan O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, Ginter CS, Carrasco N. The sodium/iodide Symporter (NIS): characterization, regulation, and medical significance. Endocr Rev. 2003;24:48–77. doi: 10.1210/er.2001-0029. [DOI] [PubMed] [Google Scholar]
- 36.Riedel C, Levy O, Carrasco N. Post-transcriptional regulation of the sodium/ iodide symporter by thyrotropin. J Biol Chem. 2001;276:21458–21463. doi: 10.1074/jbc.M100561200. [DOI] [PubMed] [Google Scholar]
- 37.Kogai T, Endo T, Saito T, Miyazaki A, Kawaguchi A, Onaya T. Regulation by thyroid-stimulating hormone of sodium/ iodide symporter gene expression and protein levels in FRTL-5 cells. Endocrinology. 1997;138:2227–2232. doi: 10.1210/endo.138.6.5189. [DOI] [PubMed] [Google Scholar]
- 38.Muscella A, Marsigliante S, Verri T, Urso L, Dimitri C, Botta G, Paulmichl M, Beck-Peccoz P, Fugazzola L, Storelli C. PKC-epsilon-dependent cytosol-to-membrane translocation of pendrin in rat thyroid PC Cl3 cells. J Cell Physiol. 2008;217:103–112. doi: 10.1002/jcp.21478. [DOI] [PubMed] [Google Scholar]
- 39.Azroyan A, Laghmani K, Crambert G, Mordasini D, Doucet A, Edwards A. Regulation of pendrin by pH: dependence on glycosylation. Biochem J. 2011;434:61–72. doi: 10.1042/BJ20101411. [DOI] [PubMed] [Google Scholar]