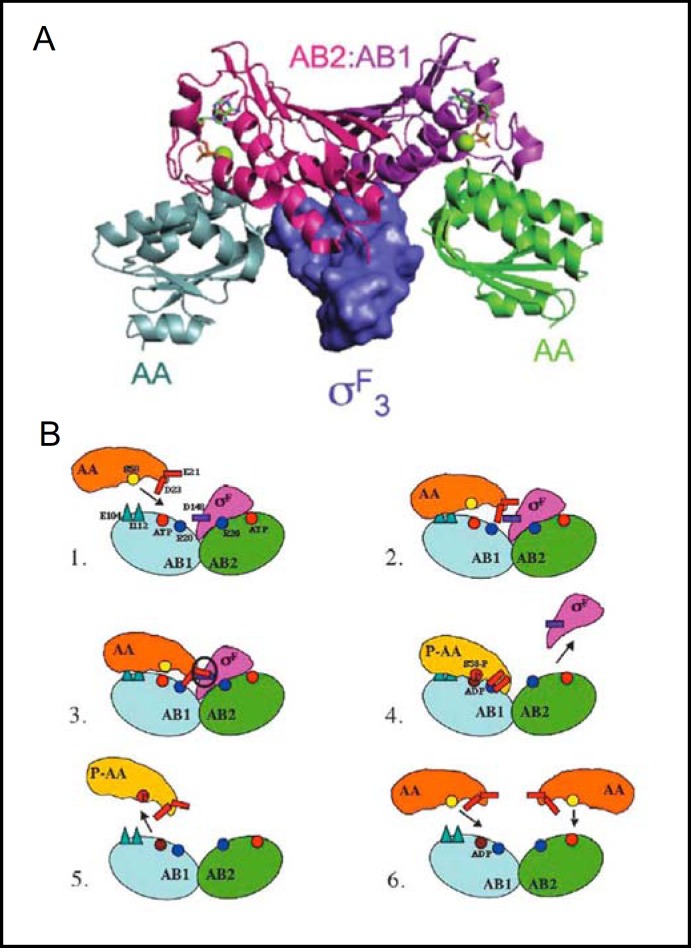
Fig. 1.
A. X-ray crystal structure of the complex of B. subtilis SpoIIAB anti-ρ homodimer kinase (comprising protomers AB1 (purple) and AB2 (magenta), with the aF domain of holo-sigma factor 0F superposed with the complex of SpoIIAB homodimer and two SpoIIAA anti-anti-ρ monomers (gray and green). Nucleotides bound to each SpoIIAB protomer are shown in green stick and active site Mg2+ as green balls. Reproduced from [9]. B. SpoIIAB catalytic cycle. Residues important for binding and dissociation are shown in (1): AB1 protomer of SpoIIAB (blue) is targeted by SpoIIAA (orange), as its docking surface (R20 in particular) is more accessible than in AB2 (green). (2) SpoIIAA binds to initial sites on SpoIIAB1 (E104, I112). (3). Bound SpoIIAA D23 interacts with SpoIIAB1 R20, leading to steric clash between SpoIIAA E21 and oF D148. (4) The steric clash promotes dissociation of oF from ADP-bound SpoIIAB. SpoIIAA then adopts a conformation that allows S58 phosphorylation (yellow circle changes to red) by SpoIIAA kinase. (5) Phospho-SpoIIAA (yellow) dissociates from ADP-bound SpoIIAB. (6) Unphosphorylated SpoIIAA can bind to SpoIIAB, forming an inhibitory complex that by blocking oF binding maintains oF in its active conformation. Reproduced from [13].