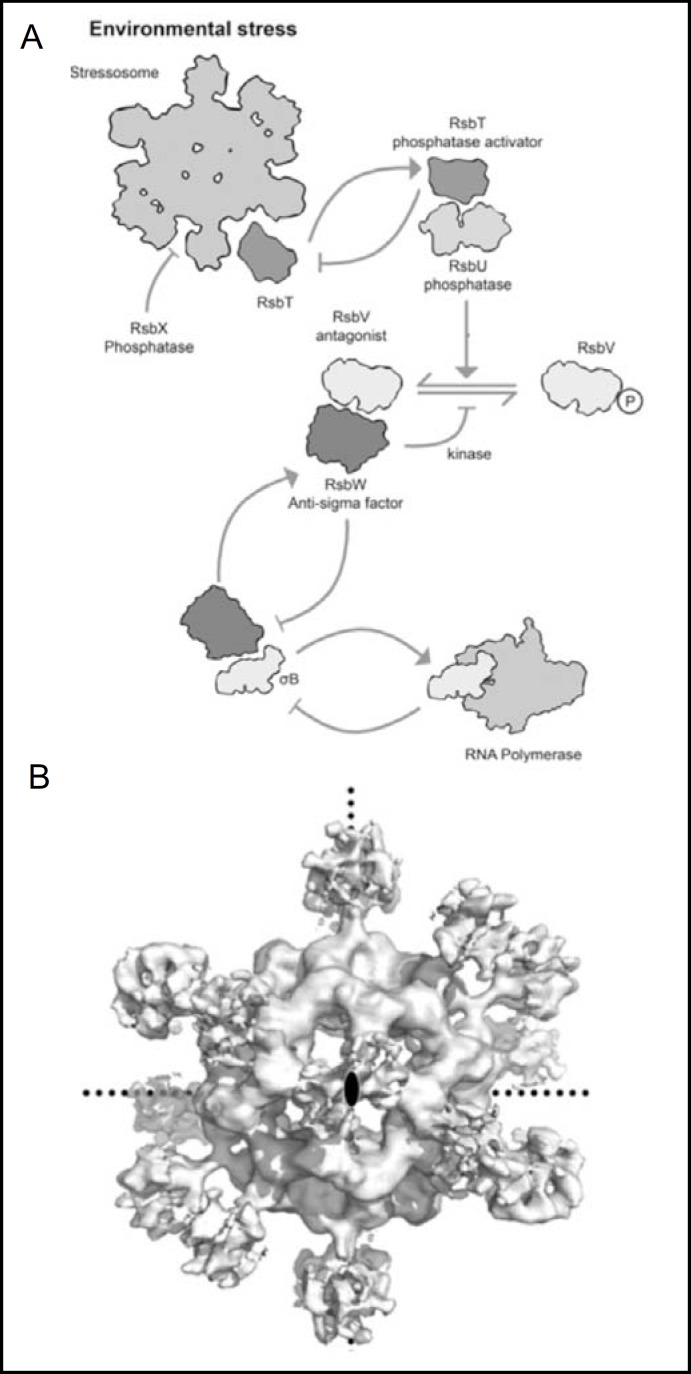
Fig. 2.
A. The crB regulatory pathway of B. subtilis. A. The 1.5 MB stressosome, an ordered 1.5 megadalton complex made up of multiple copies of STAS proteins RsbR and RsbS, serves to sequester kinase RsbT in normal conditions. Under stress, RsbT phosphorylates both STAS proteins, resulting in its release from the stressosome to bind and activate RsbU phosphatase. The RsbT/RsbU copmplex-mediated dephosphorylation of anti-anti-ρ factor STAS protein RsbV allows it to bind anti-ρ factor RsbW, liberating ρB from its inactivating complex with RsbW, and allowing activation of RNA polymerase. B. Cryo-transmission electron microscopy-derived molecular envelope of the RbsR/RbsS stressosome at 8Å resolution, viewed down one of its 2-fold axes of symmetry (central black ellipse). Dotted lines mark the other two symmetry axes. Modified from [16].