Fig. 4.
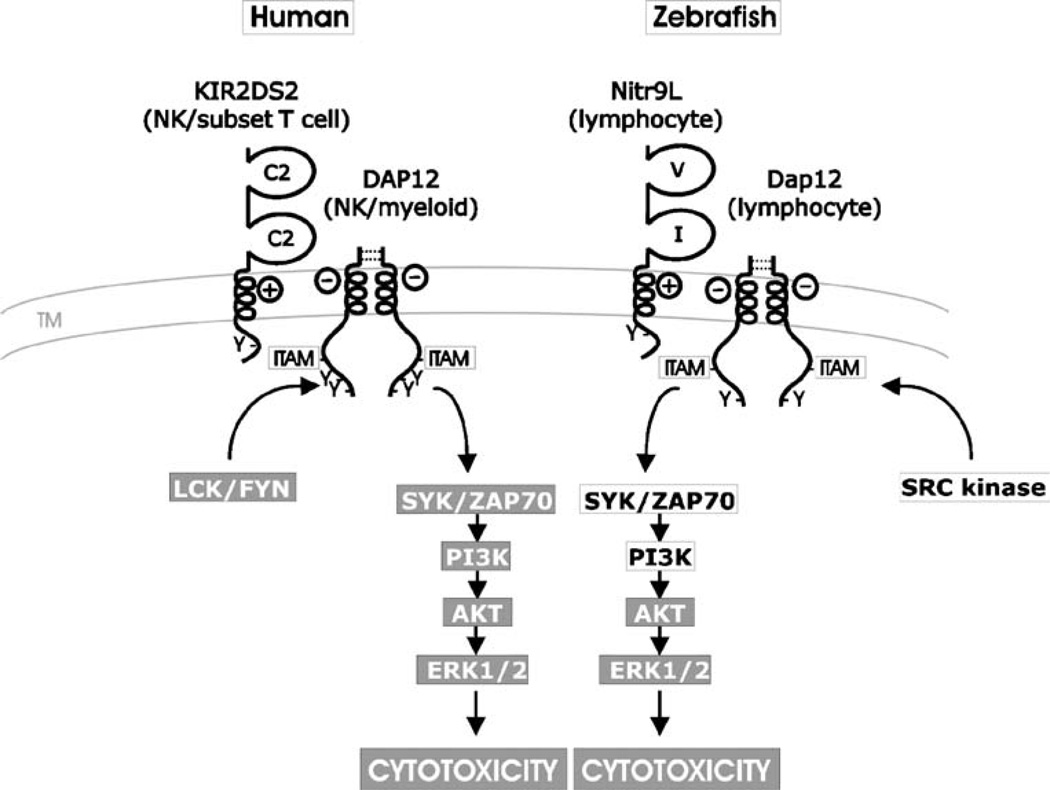
Model for Nitr9L–Dap12 activation of cytotoxic cells. The NK cell-activating pathway in humans is shown in the context of ligand recognition by the activating NK receptor KIR2DS2. DAP12 forms a dimer via two pairs of extracellular cysteines (dotted lines) and associates via complementary charges in the transmembrane domain of the activating NK receptor. Upon cross-linking, LCK/FYN phosphorylates the ITAM within the cytoplasmic tail of DAP12 that initiates the PI3K→ERK signal transduction pathway leading to cytokine release and cytotoxicity. A model for Nitr9L–Dap12 activation of cytotoxic cells parallels this process. Zebrafish Dap12 possesses the requisite cysteines for dimerization as well as the potential to interact with Nitr9L via complementary charges in transmembrane domains (Yoder et al. 2004; Yoder et al. 2007). In this study, it is shown that Nitr9L and Dap12 can physically associate and that cross-linking Nitr9L results in increased phosphorylation of AKT and ERK (Fig. 3). Components of the signaling pathway, which have been shown to be downstream of cross-linking Nitr9L/Dap12, are indicated (gray background). Cytoplasmic signaling components predicted to be associated with Nitr9L/Dap12 signaling are listed in black text
