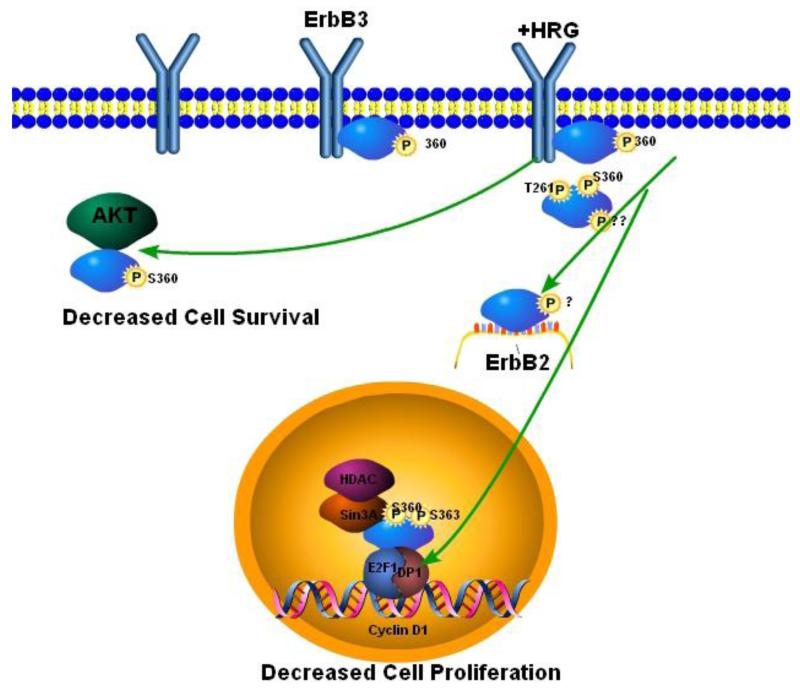
Fig. 1. Negative regulation of ErbB3 by the ErbB3 interacting proteins Ebp1 and Nrdp1.
Ebp1 phosphorylated at Ser 360 binds ErbB3 in the absence of HRG. After HRG stimulation, Ebp1 translocates to the nucleus where it becomes phosphorylated at Ser 363 and can then bind Sin3A and HDAC2 leading to repression of Cyclin D1 transcription and decreased cell proliferation. Inactivation of Ebp1 by phosphorylation at Thr 261 by Pak1 inhibits its ability to repress cyclin D1 transcription and leads to tamoxifen resistance. Ebp1 may also associate with AKT in the cytoplasm, inhibiting its activation leading to decreased cell survival. Nrdp1 can bind unphosphorylated or phosphorylated ErbB3 at the plasma membrane. Both phosphorylated and unphosphorylated receptors can be internalized. Ubiquitylation of ErbB3 in the endosome after Nrdp1 binding marks the receptors for degradation. Decreased levels of ErbB3 result in decreases in cell proliferation, AKT activation and cellular invasion. ErbB3-induced sustained activation of AKT can lead to resistance to TKIs and hormonal therapies.