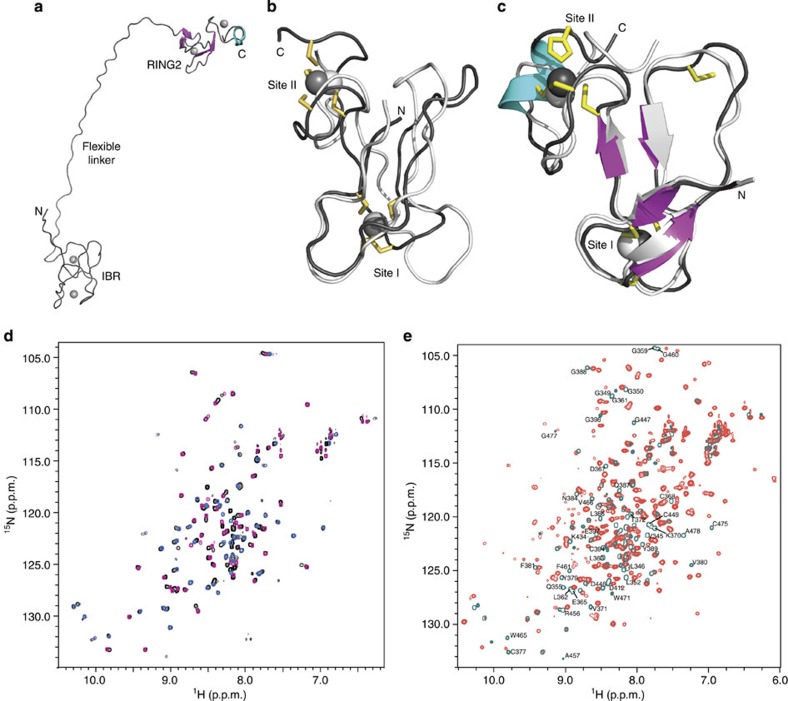
Figure 4. The structure of fly IBR–RING2 shows the domains are remote in parkin but interact with RING0 and RING1.
(a) Representative ribbon structure of parkin IBR–RING2 showing the flexible linker between the IBR and RING2 domains. (b) The superposition of parkin IBR domains from human (white, PBD accession code 2JMO) and fly (black). (c) The superposition of RING2 domains from fly parkin RING2417–482 (white) and fly parkin IBR–RING2342–482 (coloured). The two domains adopt similar folds, although the IBR domains do not contain formal β-strands. (d) Superposition of 1H–15N HSQC spectra for the fly IBR–RING2 (black contours) with spectra for the individual fly parkin IBR342–402 (pink) and RING2417–482 (blue) domains. (e) Superposition of 1H–15N HSQC spectra for parkin RING0–RING1–IBR–RING2 (red contours) and IBR–RING2342–482 (teal contours). The large number of chemical-shift changes indicates that RING0 and RING1 interact with IBR–RING2. Residues in both IBR and RING2 that undergo the largest changes in chemical shift are indicated near their positions from the IBR–RING2 assignment.