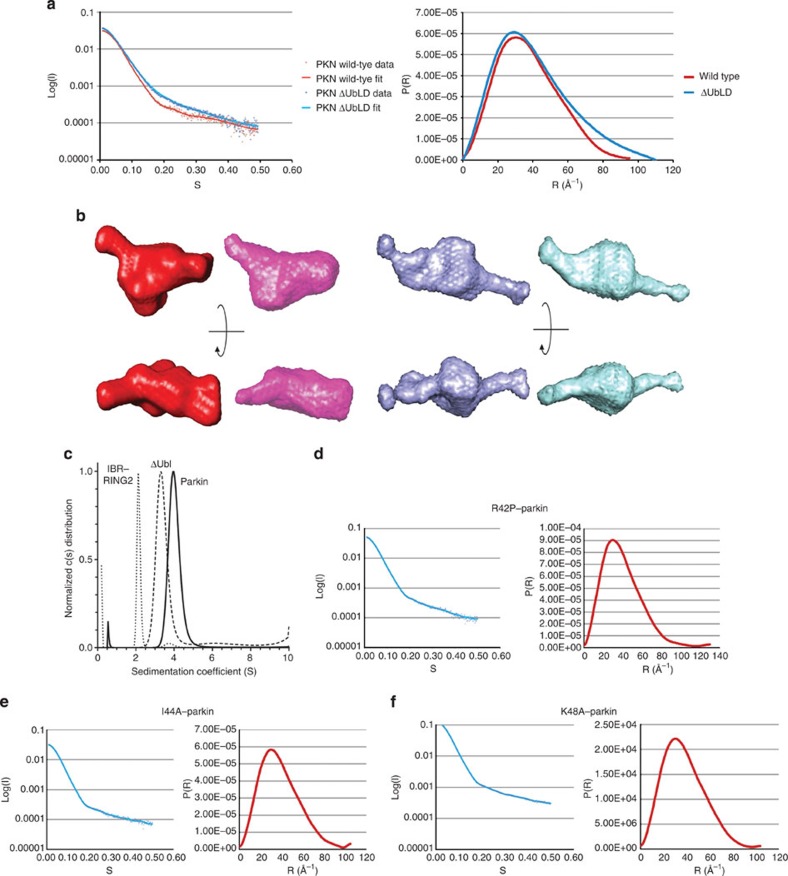
Figure 5. The tertiary structure of parkin is maintained by the Ubl.
(a) Superposition of the scattering data (left) and distance distribution (right) plots from purified human parkin samples, wild-type (red) and ΔUblD–parkin (blue). The plots show the quality of the data and the radius of gyration. (b) Representative and averaged ab initio models of wild-type parkin (red/pink) and ΔUbl–parkin (blue/cyan). Two views for each protein are shown rotated 90o about the x axis. (c) Sedimentation velocity experiments of parkin, ΔUblD–parkin, and IBR–RING2. All data were analysed using the Lamm equation and fit to a c(s) distribution. Sedimentation coefficients, corrected to 20 °C and in H2O, were determined to be 4.1 S for full-length parkin, 3.5 S for ΔUblD–parkin and 2.2 S for IBR–RING2. Fitted frictional ratios (f/f0) were calculated to be 1.38 for full-length parkin, 1.53 for ΔUbl–parkin and 1.31 for IBR–RING2. Sedimentation velocity experiments were performed at 20 °C using 10–16 μM protein in 25 mM Tris-HCl, 50 mM NaCl, 0.5 mM TCEP, pH 8.0. (d) Scattering data (left) and distance distribution (right) plots for R42P–parkin, (e) I44A–parkin, and (f) K48A–parkin.