Fig. 1.
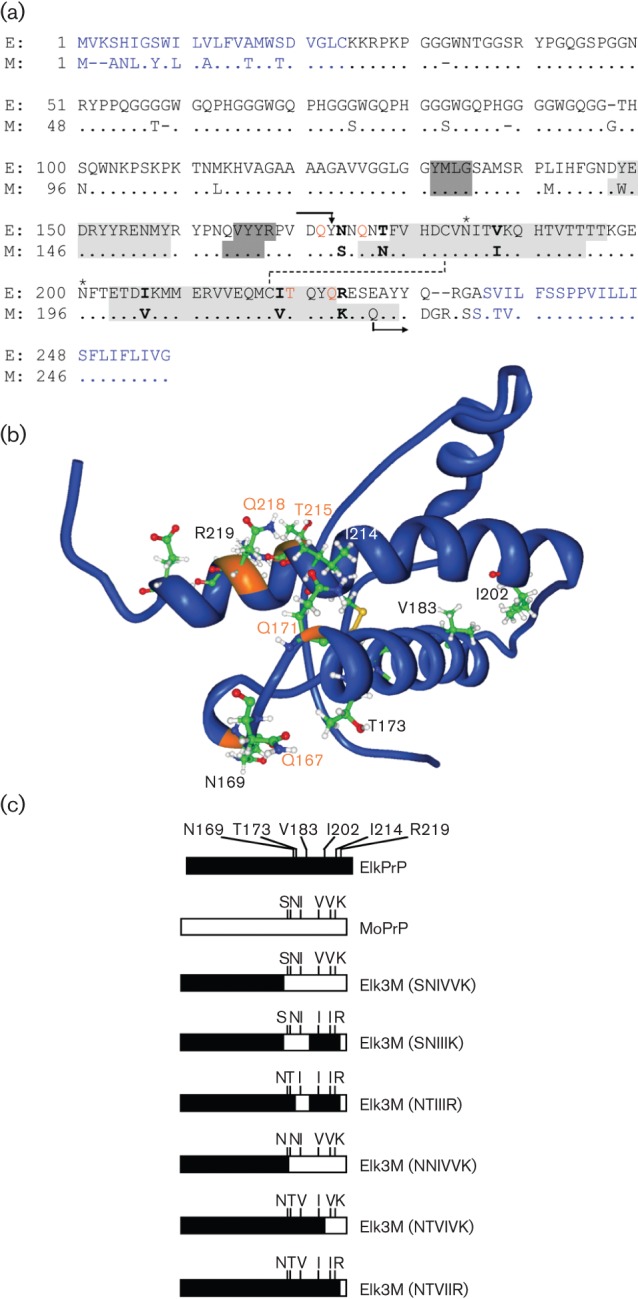
(a) Amino acid alignment of PrP from American elk (E; Cervus elaphus nelsoni, UniProt ID: P67986) and mouse (M; Mus musculus, UniProt ID: P04925). N- and C-terminal signal peptides that are cleaved off from the mature protein are indicated in blue. All chimeric PrP molecules have the N terminus of ElkPrP up to residue Y168 and the C terminus of MoPrP beginning from Q222 (mouse numbering and indicated by arrows). Between codons 169 and 221, the ElkPrP and MoPrP sequences are identical, except at six residues indicated in bold. Elk3M(SNIVVK) expresses mouse residues at these six positions; one, some, or all mutations from mouse to elk amino acids were made. Residues forming the three α-helical domains of PrP are shaded in light grey and residues forming an antiparallel β-sheet in dark grey. Glycosylation sites are indicated by asterisks; a disulfide bridge is indicated by a dashed line. Residues controlling prion replication are shown in orange (Kaneko et al., 1997; Perrier et al., 2000, 2002; Zulianello et al., 2000). (b) Nuclear magnetic resonance (NMR) structure of ElkPrP(124–234) (Gossert et al., 2005). Residues N169, T173, V183, I202, I214 and R219 (mouse numbering), which differ between elk and mouse PrP and were mutated in the chimeric PrP constructs in this study, are shown. Residues Q167, Q171, T215 and Q218, labelled in orange, are identical in elk and mouse PrP and control prion replication (Kaneko et al., 1997). (c) Schematic representation of ElkPrP (black), MoPrP (white) and chimeric elk/mouse PrP molecules expressed in Tg mice in these studies.
