Abstract
The angiotensin AT2 receptor (AT2R) has been shown to lower inflammation in the kidney. However the role of the anti-inflammatory cytokine IL-10 in AT2R mediated attenuation of inflammation has not been elucidated. We hypothesized that AT2R activation is renoprotective by directly increasing the levels of anti-inflammatory cytokine IL-10 in the kidney via nitric oxide (NO) signaling. For in vitro studies, the human proximal tubule epithelial cell-line (HK-2) was activated with lipopolysaccharide (LPS, 10 μg/ml) and/or AT2R agonist C21 (1μmol/L) for 24 hours and media cytokine levels were assessed. LPS modestly downregulated AT2R expression. Treatment with C21 lowered LPS-induced levels of both, TNF-α and IL-6 but increased IL-10 levels. Treatment with neutralizing IL-10 antibody (1 μg/ml) or NO synthase inhibitor L-NAME (1 mmol/L) abolished this effect. For in vivo studies, pre-hypertensive obese Zucker rats (OZR) and age-matched lean Zucker rats were treated for 2 weeks with C21 (300 μg/kg/day, i.p) and/or AT2R antagonist (PD123319, 50 μg/kg/min, s.c. infusion). Compared to LZR, OZR had higher levels of renal AT2R expression, TNF-α and IL-6. C21 treatment decreased levels of TNF-α by 75% and IL-6 by 60%. Conversely, PD treatment lowered the renal IL-10 levels in OZR by ~60%. Renal morphometry revealed increased mesangial matrix expansion and glomerular macrophage infiltration which was improved by C21 treatment in OZR. Our findings suggest that proximal tubule AT2R activation is anti-inflammatory by increasing IL-10 production which is largely NO-dependent and thus offers renoprotection by preventing early inflammation-induced renal injury in obesity.
Keywords: AT2 Receptor, C21, Interleukin-10, Nitric oxide, Anti-inflammatory, Renoprotection
INTRODUCTION
Obesity, insulin resistance, hypertension and renal injury are all associated with underlying low grade inflammation that results from the pro-inflammatory cytokines secreted by the adipose tissue. High levels of circulating pro-inflammatory cytokines result in immune cell infiltration and activation of resident macrophages in the kidney, which is susceptible to injury owing to obesity related excessive excretory load, hyperinsulinemia and renal lipotoxicity. Activated resident macrophages then themselves produce a host of cytokines locally, setting up a pro-inflammatory milieu, thereby accelerating the renal injury mechanisms. In addition to immune cells, the proximal tubule epithelial cells (PTECs) are known to play an important role in renal inflammation by producing an array of chemokines and cytokines. Among these, tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6) and interleukin-10 (IL-10) are key players in mediating renal inflammation and injury1. Typically, the anti-inflammatory cytokine IL-10 negatively regulates pro-inflammatory cytokine signaling to maintain homeostasis2. This self-regulatory system is rendered dysfunctional in obesity/diabetes3 due to a skewing of macrophages to the pro-inflammatory phenotype. This unresolved inflammation makes the kidney more susceptible to obesity-related glomerular hyperfilteration, glomerular cell proliferation, matrix accumulation, basement membrane thickening and ultimately glomerulosclerosis and tubular fibrosis culminating in nephron loss.
In addition to creating a pro-inflammatory environment, obesity also leads to an abnormally activated renin angiotensin system (RAS), an important hormonal system involved in regulating renal structure and function. Earlier, we and others have reported in obese Zucker rats, an increased function of the renal angiotensin type 1 receptor (AT1R) 4, 5, 6 which mediates most of the deleterious effects of Angiotensin II (Ang II), including the pro-inflammatory functions7, 8, 9, 10, 11, 12. In addition, there is an increase in the renal angiotensin type 2 receptor (AT2R) expression in these animals. The AT2R is believed to be protective by functionally antagonizing the pro-inflammatory actions of Ang II mediated by AT1R, via multiple signaling pathways such as NO/cGMP13, 14, 15 and activation of tyrosine phosphatases16, 17.
Recently, it has been demonstrated that acute AT2R stimulation is renoprotective by lowering the renal levels of TNF-α and IL-6 in a rat model of renovascular hypertension18. Consistent with this report, we also have previously shown that chronic AT2R activation in hypertensive obese Zucker rats (OZR) lowers blood pressure as well as circulating and renal levels of pro-inflammatory cytokines TNF-α, IL-6 and MCP-119. Further, Curato et al.20 have recently identified a population of non-cytotoxic AT2R expressing CD8+ T-cells with a unique phenotype of increased IL-10 expression, suggesting that the AT2R may elicit its anti-inflammatory response by promoting IL-10 production. However, the role of AT2R in renoprotection in obesity independent of blood pressure change and the involvement of proximal tubule AT2Rs in mediating anti-inflammation are not known. We hypothesized that AT2R activation is renoprotective by increasing the levels of the anti-inflammatory cytokine IL-10 in the kidney via nitric oxide (NO) signaling. Here, we demonstrate that proximal tubule AT2R stimulation attenuates inflammation by increasing IL-10 production, which is largely NO-dependent. Activation of the AT2R is therefore renoprotective since this prevents early inflammation-induced renal injury in obesity.
METHODS
Cell Culture
Human kidney (HK-2) proximal tubule epithelial cells were cultured according to standard protocols. For detailed protocols please refer to the online data supplement.
In Vitro Experimental Protocols
TNF-α, IL-6 and IL-10 were quantified in the media from HK-2 cells 24 hours after stimulation with LPS (10 μg/ml) and/or C21 (1 μmol/L). For detailed protocols please refer to the online data supplement.
Animal Model
Male, 5 week old lean and pre-hypertensive obese Zucker rats (LZR and OZR, respectively) were used (n=6-7). These animals were treated with AT2R agonist (C21 300 μg/kg/day i.p. daily) and/or antagonist (PD123319 50 μg/kg/min s.c infusion) for 2 weeks to determine the effect of AT2R activation or blockade on obesity-linked renal inflammation and injury. Renal injury was assessed using periodic acid Schiff (PAS) stained sections of the kidney according to the method described by Raij et al.21. Macrophage infiltration was determined by immunostaining for CD68, a monocyte/macrophage marker. For detailed protocols please refer to the online data supplement.
Enzyme-linked Immunosorbent Assay (ELISA)
Cytokines in cell-culture supernates and kidney cortex homogenates were assessed by kit-based ELISA according to the manufacturer’s instructions. For detailed protocols please refer to the online data supplement.
Immunoblotting
Western blotting for plasma cytokines (TNF-α, IL-6 and IL-10) and angiotensin receptors (AT1 and AT2) in kidney cortex homogenates and HK-2 cells was carried out according to standard techniques. Amount of protein loaded for rat samples was 20 μg AT1R and 60 μg for AT2R expression. For detailed protocols please refer to the online data supplement.
Statistical Analyses
Data are expressed as mean±SEM with n > 5 in each group. Student’s t-test was used. One-way ANOVA with post-hoc (Newman-Keuls) tests was used to compare variations within groups. A value of p<0.05 was considered statistically significant.
RESULTS
In vitro studies
Effect of AT2R agonist C21 on cytokine production by activated PTECs
HK-2 cells were treated with bacterial lipopolysaccharide (LPS, 10μg/ml) for 24 hours to induce cytokine production in PTECs. Another set of cells were treated with AT2R agonist C21 (1 μmol/L) along with LPS to determine the effect of AT2R stimulation on cytokine production by activated PTECs. Treatment with LPS downregulated AT2R expression (see supplemental results in data supplement), which is consistent with reports in other tissues 22, 23. Further, LPS treatment alone resulted in a ~50-fold increase in TNF-α and ~10-fold increase in IL-6 concentration in the media. Concurrent treatment with C21 lowered TNF-α concentration by ~70% and IL-6 concentration by ~60% (Fig. 1A-B). In addition to LPS, in a separate set of experiments, PTECs were activated using TNF-α (10 ng/ml) for 24 hours and IL-6 production in the media was determined. Similar to LPS, TNF-α aggravated the production of IL-6 by ~10- fold, which was lowered by ~50% with concurrent treatment C21 treatment (See supplemental results in data supplement). Predictably, LPS treatment increased IL-10 production in HK-2 cells, but not to the same extent as C21 treatment alone. Further, treatment with LPS and C21 together resulted in greater IL-10 levels in the media compared to LPS treatment. However, this was not significantly different from the IL-10 production by C21 treatment alone (Fig. 1C). Activation of PTECs with TNF-α with and without C21 followed a pattern of IL-10 production similar to that observed with LPS treatment (See supplemental results, Fig. S3). C21 treatment alone did not alter pro-inflammatory cytokines, TNF-α and IL-6 production by PTECs. On the other hand, C21 treatment alone dose-dependently (0.1-10 μmol/L) increased the production of IL-10 in PTECs, even in the absence of LPS activation (See supplemental results, Fig S4).
Fig. 1.
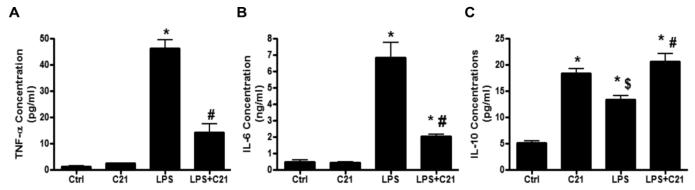
Concentration of (A) tumor necrosis factor-α (TNF- α), (B) interleukin-6 (IL-6) and (C) interleukin-10 (IL-10) in the media collected from HK-2 proximal tubule epithelial cells after activation with lipopolysaccharide (LPS, 10μg/ml) and/or AT2R agonist (C21, 1μmol/L) for 24 hours. Cytokine concentrations in media were measured by ELISA. Data are represented as mean ± SEM. * indicates p<0.05 vs control, # indicates p<0.05 vs LPS treated and $ indicates p<0.05 vs C21 treated HK-2 cells (n=7).
Effect of neutralizing IL-10 antibody on cytokine production by activated PTECs
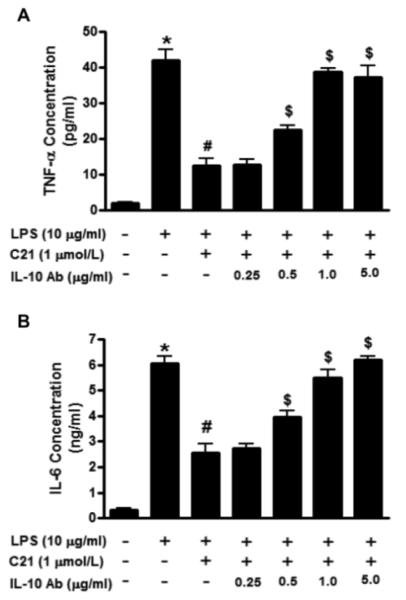
HK-2 cells were treated with neutralizing antibody to IL-10 which binds to IL-10 produced by these cells and prevents it from interacting with its receptor. Prior to treatment with LPS and C21, the cells were pre-incubated for 30 mins with different doses (0.25, 0.5, 1 and 5 μg/ml) of the neutralizing IL-10 antibody. The IL-10 antibody was able to dose-dependently abolish the ability of the AT2R agonist to lower TNF-α and IL-6 (Fig. 2A and 2B).
Fig. 2.
Effect of increasing concentrations of neutralizing interleukin-10 (IL-10) antibody (0.25, 0.5, 1, 2.5 μg/ml) on the concentration of (A) tumor necrosis factor-α (TNF- α) and (B) interleukin-6 (IL-6) in the media collected from HK-2 proximal tubule epithelial cells after activation with lipopolysaccharide (LPS, 10μg/ml) and/or AT2R agonist (C21, 1μmol/L) for 24 hours. Cytokine concentrations in media were measured by ELISA. Data are represented as mean ± SEM. * indicates p<0.05 vs control, # indicates p<0.05 vs LPS treated and $ indicates p<0.05 vs LPS+C21 treated HK-2 cells (n=7).
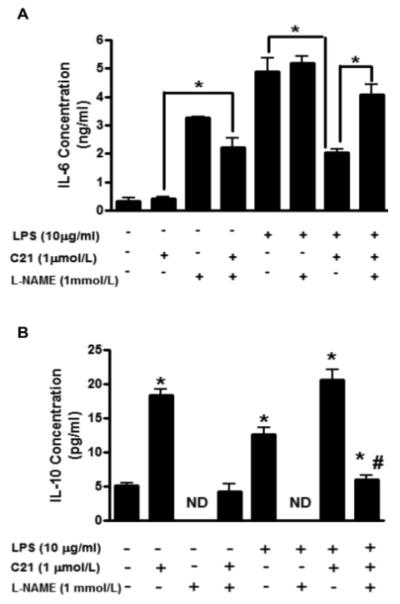
Effect of L-NAME on cytokine production by PTECs
HK-2 cells were pre-incubated for 15 min with nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME; 1 mmol/L) prior to treatment with LPS and/or C21. Incubation with L-NAME alone led to a 3-fold increase in the levels of IL-6 released in the medium. In cells pre-incubated with L-NAME, treatment with C21 also led to a similar increase in IL-6 production compared to control C21 treated cells. In the presence of L-NAME + LPS treated cells, there was no significant difference in the IL-6 production compared to control LPS activated cells. However, the attenuation of IL-6 levels by C21 in LPS-activated PTECs was lost in the cells where L-NAME was added (Fig.3A). On the other hand, L-NAME alone significantly lowered the IL-10 production in HK-2 cells. There was also a significant inhibition in the C21 as well as LPS-mediated IL-10 production in the presence of L-NAME. Further, the increase in IL-10 levels in C21 treated LPS activated PTECs was completely abrogated in the presence of NOS inhibitor (Fig. 3B). Taken together, these data indicate that the anti-inflammatory response to AT2R activation is largely dependent on AT2R mediated NO production.
Fig. 3.
Effect of nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME) on the concentration of (A) interleukin-6 (IL-6) and (B) interleukin-10 (IL-10) in the media collected from HK-2 proximal tubule epithelial cells after activation with lipopolysaccharide (LPS, 10μg/ml) and/or AT2R agonist (C21, 1μmol/L) for 24 hours. Cytokine concentrations in media were measured by ELISA. Data are represented as mean ± SEM. * indicates p<0.05 vs respective control (A), # indicates p<0.05 vs LPS+C21 (B) treated HK-2 cells (n=5). ND indicates not detected.
In vivo studies
Body weight and blood pressure
At age 7 weeks, the OZRs weighed significantly more than LZRs and drug treatment did not alter body weight in any of the experimental groups (Lean: Veh 202±3g, PD 210±4.5g, C21 204±6g, PD+C21 199±8g vs Obese: Veh 348±10.5g, PD 348±9.6g, C21 345±9g, PD+C21 363±11g). Blood pressure was measured in conscious animals weekly by tail cuff plethysmography (CODA Non-invasive Blood Pressure System, Kent Scientific, Torrington, CT). The systolic BP in OZR was comparable to lean controls throughout the duration of the study and drug treatment did not affect the BP in any of the experimental groups (Lean: Veh 105±1.7 mmHg vs Obese: Veh 106±1.5 mmHg, PD 104±2.1 mmHg, C21 103±0.5 mmHg 102±1.5 mmHg).
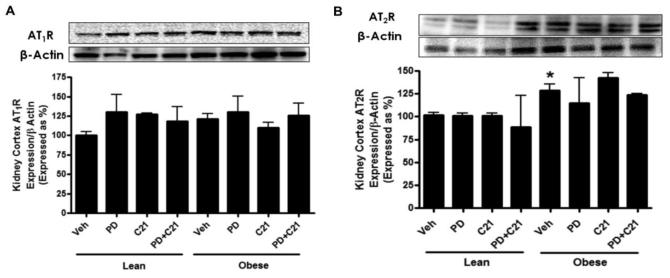
AT1 and AT2 receptor expression in renal cortex
A distinct band was detected for AT1R at ~41 kDa by western blotting. Densitometric analysis revealed that AT1R expression was not significantly different in the renal cortex of control OZR compared to control LZR. Treatments did not affect the expression levels of the AT1R in either strain of rats (Fig. 4A). Two bands were detected for AT2R at approximately 44 and 39 kDa in the renal cortex, most likely due to varying degrees of glycosylation24. We have previously reported that treatment of the cortical membranes with the deglycosylating enzyme N-glycanase shifted AT2R multiple bands towards a single band at ~30kDa4. AT2R expression was ~ 45% higher in OZR compared to control LZR. Drug treatments did not cause significant changes in AT2R expression in any of the experimental groups (Fig. 4B).
Fig.4.
Expression of angiotensin (A) AT1 (AT1R) and (B) AT2 receptors (AT2R) in the kidney cortex of lean and prehypertensive obese Zucker rats after 2 weeks treatment with vehicle (Veh), AT2R antagonist PD123319 (PD), AT2R agonist (C21) and both PD123319+C21 (PD+C21). The upper panels show representative western blots for AT1R, AT2R and β-actin. Lower panels represent quantitative results normalized to β-actin. Data are mean ± SEM. * indicates p<0.05 vs lean vehicle treated rats (n=5-6).
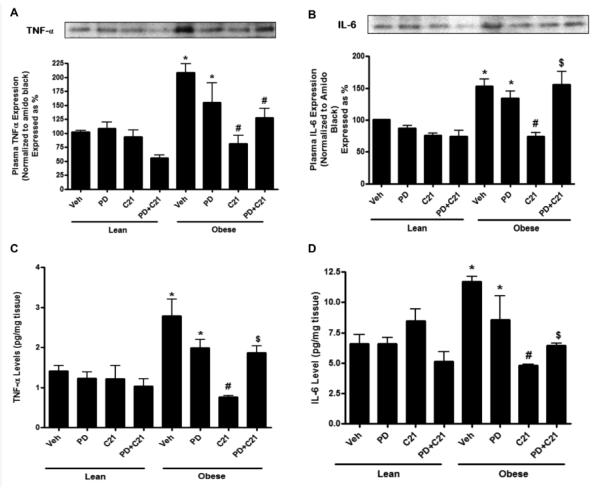
Pro-inflammatory cytokine production in response to PD and C21
Compared to control LZR, the levels of pro-inflammatory cytokines TNF-α and IL-6 were higher in plasma as well as renal cortex in control OZR. These levels were significantly reduced with AT2R agonist treatment for 2 weeks. PD treatment itself did not alter the pro-inflammatory TNF-α and IL-6 cytokine levels; however, it was able to prevent the C21 mediated lowering of pro-inflammatory cytokines (Fig. 5A-D) indicating that the anti-inflammatory effect of C21 was indeed via the AT2R. Drug treatment did not have any effect on the cytokine levels in LZR.
Fig. 5.
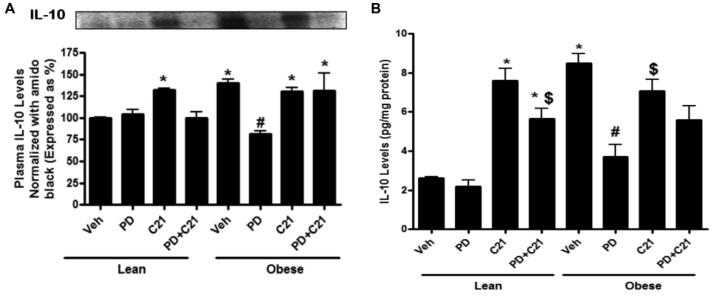
Pro-inflammatory cytokine expression in the plasma (A,B) and concentration in the kidney cortex (C,D) of lean and pre-hypertensive obese Zucker rats after 2 weeks treatment with vehicle (Veh), AT2R antagonist PD123319 (PD), AT2R agonist (C21) and both PD123319+C21 (PD+C21). (A) Tumor necrosis factor- α (TNF- α) and (B) Interleukin-6 (IL-6) expression in the plasma normalized with total plasma protein stained with amido black. Top panels show representative western blots for TNF- α and IL-6 (C) TNF-α and (D) IL-6 levels in the kidney normalized to total protein. Data are mean ± SEM. * indicates p<0.05 vs lean vehicle treated, # indicates p<0.05 vs obese vehicle treated and $ indicates p<0.05 vs obese C21 treated rats (n=6).
Anti-inflammatory cytokine production in response to PD and C21
Compared to control LZR, OZR had higher IL-10 levels in both, plasma and kidney cortex and this was not altered by C21 treatment. On the other hand, PD treatment resulted in ~45% lowering of circulating and ~60% reduction in the kidney IL-10 levels in OZR. Treatment with PD had no effect on IL-10 levels in LZR, however, C21 treatment in LZR led to a 3-fold increase in IL-10 levels in the plasma and renal cortex (Fig. 6A-B). Taken together, these data point towards a role of AT2R in IL-10 production.
Fig. 6.
Anti-inflammatory cytokine interleukin-10 (IL-10) expression in the plasma (A) and concentration in the kidney cortex (B) of lean and pre-hypertensive obese Zucker rats after 2 weeks treatment with vehicle (Veh), AT2R antagonist PD123319 (PD), AT2R agonist (C21) and both PD123319+C21 (PD+C21). (A) IL-10 expression in the plasma was normalized with total plasma protein stained with amido black. Top panels show representative western blots for IL-10. (B) IL-10 levels in the kidney normalized to total protein. Data are mean ± SEM. * indicates p<0.05 vs lean vehicle treated, # indicates p<0.05 vs obese vehicle treated and $ indicates p<0.05 vs obese C21 treated rats (n=6).
Renal Morphological Analysis
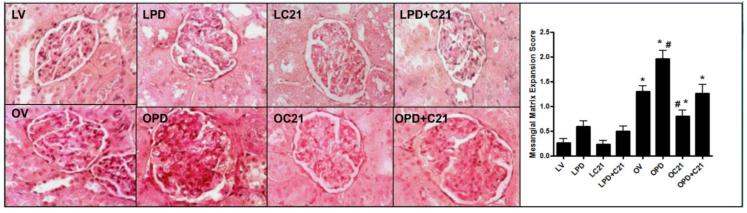
LZRs exhibited normal renal morphology and this was not affected by drug treatments (Fig. 7A). Control OZR had higher (1.3±0.2) mesangial matrix expansion (MME) scores compared to LZR, which had a MME score below 0.5 indicating intact renal structure. This was worsened by PD treatment; these animals had MME scores of ~2 which is indicative of damage involving 25-50% of the affected glomerulus. OZRs treated with C21 were protected from this early event associated with obesity-linked renal pathology and had near normal MME scores (Fig 7B).
Fig. 7.
Renal morphometry (A) Periodic Acid Schiff (PAS) stained sections from the kidney cortex of lean (L, upper panel) and obese (O, lower panel) Zucker rats after 2 weeks treatment with vehicle (V), AT2R antagonist PD123319 (PD), AT2R agonist (C21) and both PD123319+C21 (PD+C21). (B) Semi-quantitative mesangial matrix expression (MME) score from PAS stained sections. An average MME score was obtained from analysis of 30 glomeruli per rat. Data are mean ± SEM (n=6). * indicates p<0.05 vs lean vehicle treated, # indicates p<0.05 vs obese vehicle treated rats (400x magnification).
Effect of C21 and PD treatment on renal macrophage infiltration
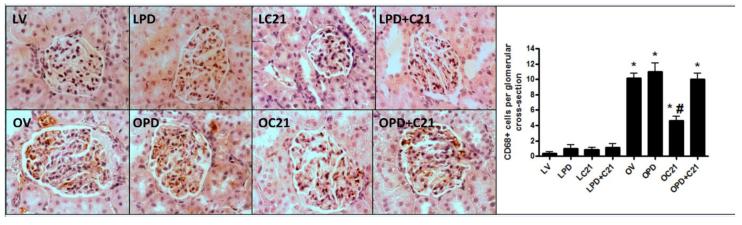
LZR exhibited almost no macrophage infiltration (Fig. 8A) irrespective of drug treatments. Compared with LZR, control OZR demonstrated increased CD68+ cells in the glomeruli (0.3±0.2 vs 10±0.7) and this was attenuated (10±0.7 vs 4.7±0.5) by C21 treatment (Fig 8B).
Fig. 8.
Monocyte/macrophage infiltration in glomeruli (A) CD68 immunostained sections from the kidney cortex of lean (L, upper panel) and obese (O, lower panel) Zucker rats after 2 weeks treatment with vehicle (V), AT2R antagonist PD123319 (PD), AT2R agonist (C21) and both PD123319+C21 (PD+C21). (B) Quantitative measurement of CD68+ monocyte/macrophages from immunostained sections. An average number of macrophage infiltration was obtained from analysis of 30 glomeruli per rat. Data are mean ± SEM (n=6). * indicates p<0.05 vs lean vehicle treated, # indicates p<0.05 vs obese vehicle treated rats (400x magnification).
DISCUSSION
Non-resolving renal inflammation, which begins with infiltration of mononuclear cells in the kidney, is one of the earliest events in progressive renal injury that finally culminates in glomerulosclerosis and tubulointerstitial fibrosis25, 26. In addition to the immune cells, the proximal tubule epithelial cells (PTECs) have recently been shown to be protective and can inhibit the activation of macrophages27. Also, PTECs have been shown to express the AT2R4, 28, 29. In the present study, LPS-activated HK-2 cells produced significant amounts of pro-inflammatory cytokines TNF-α and IL-6, which led to a compensatory increase in IL-10 production. Treatment with selective AT2R agonist C21 resulted in lower levels of TNF-α and IL-6 in the media collected from LPS-activated HK-2 cells, which is in agreement with previous reports in dermal fibroblasts30. However, here we also observed a marked increase in the IL-10 levels when LPS activated cells were treated with C21. Interestingly, while treatment with C21 alone did not alter the production of pro-inflammatory cytokines, there was a significant increase in IL-10 levels with C21 even in the absence of LPS. Furthermore, this increase was greater than what was observed with LPS-activation, indicating that AT2R activation directly drives the production of IL-10, possibly independent of classical toll-like receptor mediated pathways. Moreover, the lowering of pro-inflammatory cytokines by C21 was completely lost in the presence of a neutralizing antibody to IL-10, implying that even a modest increase in IL-10 is essential for the lowering of pro-inflammatory cytokine levels by AT2R agonist. Overall, these results suggest that IL-10 is the dominant cytokine responsible for mediating the response to AT2R agonist. In addition to using LPS to activate PTECs, in another set of experiments, TNF-α also was employed to stimulate the production of cytokines (see online data supplement). This ensured that the anti-inflammatory response to AT2R agonist was not specific to toll-like receptor signaling. Moreover, in obesity-associated renal inflammation, TNF-α from the plasma as well as infiltrating macrophages is likely to activate PTECs. However, since the results obtained by both activating agents were similar, the subsequent experiments were conducted using LPS where the outcomes were more remarkable.
Although the anti-inflammatory role of AT2R stimulation has recently been ascertained by multiple groups18, 19, 30, the precise molecular mechanisms that produce this response are still under investigation. A few signaling pathways by which the AT2R may potentially lower the levels of pro-inflammatory cytokines have been described, including inhibition of NFκB, activation of protein phosphatases, increase in epoxyeicosatrienoic acid synthesis30 and inhibition of STAT3 phosphorylation31. Nitric oxide (NO) is known to be a key second messenger in renal AT2R signaling13, 14, 15. Additionally, the anti-inflammatory properties of physiological levels of NO have been described32, 33. In the in vitro studies here, it was clear that the non-specific NOS inhibitor, L-NAME, inhibited the production of IL-10 in LPS and/or C21 treated PTECs, while at the same time, the levels of IL-6 were elevated in LPS activated cells even in the presence of C21. Although the precise link between NO production and IL-10 signaling is still unclear, it can be speculated that the signaling cascades activated by NO, including cGMP-dependent protein kinase (PKG) activation may be involved in downstream activation of MAPKs34 that are required for IL-10 production.
Resolution of renal inflammation is essential for the control of renal injury initiated by obesity. In order to demonstrate that AT2R agonist can attenuate early changes associated with obesity-linked renal inflammation independent of hypertension, we used pre-hypertensive obese Zucker rats (OZR) as the animal model. The OZR is a well characterized model of metabolic syndrome and develops spontaneous renal injury with increasing age35, 36, 37. At age 5 weeks these animals are obese and hyperinsulinemic and develop hyperglycemia and hypertension after 9 weeks of age. These animals also exhibit hyperfiltration, however the relative contribution of the pro-inflammatory cytokines to this observation is difficult to assess, since hyperfiltration is a cumulative effect of multiple factors including high body mass index, increased excretory load, hyperinsulinemia and inflammation and oxidative stress. We have previously shown that 2 week treatment with AT2R agonist CGP42112A in 12 week old OZR was able to lower plasma and renal levels of TNF-α, IL-6 and MCP-119 as well as lower blood pressure. Here, we demonstrate that pre-hypertensive OZRs also have up-regulated renal AT2R expression and elevated plasma cytokines. Similar to our previous findings, 2 weeks treatment with AT2R agonist C21 in this age group resulted in marked decline in the TNF-α and IL-6 levels in the plasma and the kidney cortex, but without any change in blood pressure. This observation, when considered along with the in vitro data makes it evident that activation of the renal AT2R has a direct anti-inflammatory effect. Also, in our previous studies, it was reported that there was an increase in TNF-α in the plasma of lean rats treated with CGP. This was not observed in the present study using C21. This disparity in observations may be a result of different AT2R agonists used, since CGP has been reported to have some non-AT2R mediated effects as well38, 39, while C21 is believed to be more AT2R selective.
Renal morphometry revealed an increase in macrophage infiltration, glomerular hypertrophy, mesangial matrix expansion (MME) and basement membrane thickening in control OZR compared to lean controls. There was no focal segmental glomerulosclerosis (FSGS) or any extent of significant tubular fibrosis detected, which is not surprising, since at this age it is unlikely that such drastic nephron damage would occur. Also, OZRs have been documented to have normal renal function at age 6 weeks and only develop proteinurea at 12-14 weeks of age and decreased GFR at 32 weeks of age35, 40. Thus, in the age group used in this experiment no significant changes in renal function were expected. Nevertheless, leukocyte infiltration and MME are still among the earliest pathophysiological changes that are observed in the kidney in obesity-linked renal damage, which predispose to FSGS and tubulointerstitial fibrosis25, 37. Treatment with AT2R antagonist PD worsened the extent of MME and macrophage infiltration, while C21 treatment maintained the renal structural integrity in OZRs. Drug treatment in LZR had no effect on renal morphology in any of the experimental groups, emphasizing the fact that AT2R plays a more significant role in pathophysiological conditions rather than in the normal state. In agreement with our in vitro data, the in vivo studies also revealed that AT2R is involved in anti-inflammatory cytokine production. However, in animals, we observed that OZRs already had elevated levels of the anti-inflammatory IL-10, which differs from some published reports in humans where obesity was associated with lower circulating levels of IL-1041, 42. It is likely that the levels of IL-10 were increased in the early stages of obesity linked inflammation in response to the high levels of TNF-α as a compensatory mechanism. These already high levels of plasma and renal cortical IL-10 were not further increased by C21 treatment, possibly because the IL-10 levels were at the maximal production limit. We did, however, see a marked reduction in the levels of this cytokine when the AT2R was blocked with PD; making it clear that increase in anti-inflammatory cytokine production is AT2R-mediated. A possible reason for the difference in these findings from those observed in the HK-2 cells might be that in obese animals, the anti-inflammatory cytokines are already at their maximal levels, and hence, the increase caused due to AT2R agonist may not be readily evident. Also, unlike the pro-inflammatory cytokines, which were unaffected by drug treatments, C21 and PD, both caused significant alterations in the levels of IL-10 in the kidney cortex in lean rats, which suggests that AT2R activation may have a direct role in increasing IL-10 production.
In this study, we have demonstrated a ~45% increase in renal AT2R expression in OZR compared to LZR. While this is a modest increase in receptor expression, the responses to AT2R agonist and antagonist are quite impressive in comparison. It is important to note that in case of the AT2R, the function of this receptor has been shown to be enhanced in OZR compared to LZR, as we have reported previously 4, 43. Furthermore, it has been reported in rat proximal tubules that AT2R activation itself directly lowers AT1R function via the NO/cGMP/Sp1 pathway44, revealing an additional mechanism which could contribute to the physiological response to AT2R agonist. Thus, receptor expression alone is not sufficient to account for the changes in the response to AT2R agonist and antagonist, since post-signaling amplification can play a major role in the net response elicited by receptor activation.
Overall, the most noteworthy finding of the in vivo and in vitro studies is the involvement of IL-10 in mediating the anti-inflammatory action of the AT2R, which adds another dimension to the existing paradigm where AT2R has been shown to merely lower the pro-inflammatory cytokine levels. Although the effect of C21 on the entire network of cytokines and chemokines has not been investigated in this study, the effects on the key cytokines make it evident that the AT2R does elicit an anti-inflammatory action, which is independent of its anti-hypertensive effect. Here, we have shown the beneficial effects of activating the PTECs using HK-2 cells; however, in whole animals the situation is much more complex, since there are a number of other cell-types, such as the mesangial cells, the podocytes and the endothelial cells, which express the AT2R in addition to producing cytokines. So the net anti-inflammatory effect observed in OZRs is most likely a result of all these factors, underscoring the importance of the intra-renal AT2R in renoprotection. We conclude that chronic AT2R activation is renoprotective by increasing intra-renal IL-10 production via increased NO signaling.
PERSPECTIVES
These in vitro and in vivo studies could help to elucidate the complex AT2R-mediated anti-inflammatory and renoprotective mechanisms and identify it as a novel therapeutic target for preventing the onset and progression of obesity-associated kidney disease.
Supplementary Material
Fig. S1: AT1R Expression in HK-2 cells. Representative western blot for AT1R (~41 kDa) showing a detectable band with both 10 μg and 50 μg of loaded protein.
Fig. S2: AT2R Expression in HK-2 proximal tubule epithelial cells. Representative western blots for AT2R protein (approx. 40 and 45 kDa) top panel and for β–actin in the lower panel. Data are mean ± SEM (n = 3). * indicates p<0.001 vs control untreated HK-2 cells.
Fig. S3: (A) Interleukin-6 (IL-6) and (B) interleukin-10 (IL-10) concentration in the media collected from HK-2 cells stimulated by TNF-α (10 ng/ml) and/or C21 (1 umol/L). Data are mean ± SEM (n = 3). * indicates p<0.05 vs control untreated, # indicates p<0.05 vs TNF-α treated and $ indicates p<0.05 vs C21 treated HK-2 cells.
Fig. S4: Dose dependent increase in interleukin-10 (IL-10) production by HK-2 proximal tubule epithelial cells following treatment with AT2R agonist (C21; 0.1-10 μmol/L) for 24 hours. AT2R antagonist PD123319 (PD, 10 μmol/L) was able to block the C21-mediated increase in IL-10 concentration. Cytokine concentrations in media were measured by ELISA. Data are represented as mean ± SEM (n=5).
Fig. S5: Dose dependent increase in total nitrates in the media collected from HK-2 proximal tubule epithelial cells following treatment with AT2R agonist (C21; 0.1-10 μmol/L) for 24 hours. AT2R antagonist PD123319 (PD, 10 μmol/L) was able to block the C21-mediated increase in total nitrate production. Data are represented as mean ± SEM (n=5).
Fig. S6: Representative blots depicting the relative abundances of AT1R and AT2R in lean and obese Zucker rats.
NOVELTY AND SIGNIFICANCE 1) What is New, 2) What is Relevant?
What is New?
This is the first report demonstrating that the activation of the AT2R increases interleukin-10 production in HK-2 proximal tubule epithelial cells and Zucker rats. Moreover, IL-10 is the dominant cytokine involved in mediating the anti-inflammatory response of AT2R agonist.
We demonstrate that chronic AT2R activation exerts an anti-inflammatory response independent of hypertension in obese Zucker rats.
Treatment with AT2R agonist C21 for 2 weeks protects against the early obesity-linked renal injury.
What is Relevant?
Since the AT2R agonist can preserve intact renal structure and function, which may protect against obesity-linked hypertension.
Anti-inflammatory activity of AT2R agonist may itself protect against the development of hypertension in obesity.
Summary
In this study we demonstrate that activation of the AT2R lowers the levels of pro-inflammatory cytokines in the kidney and this anti-inflammatory response is mediated by a NO-dependent increase in IL-10 production. Chronic activation of the AT2R for 2 weeks with AT2R agonist, C21, is renoprotective since it prevents the development of early macrophage infiltration and mesangial matrix expansion in obese Zucker rats.
Acknowledgments
SOURCES OF FUNDING This study was supported by grant R01 DK-61578 from the National Institutes of Health to TH
Footnotes
CONFLICT OF INTREST/ DISCLOSURE STATEMENT None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Girndt M, Kohler H, Schiedhelm-Weick E, Schlaack JF, Meyer zum Buschenfeld KH, Fleischer B. Production of interleukin-6, tumor necrosis factor alpha, and interleukin-10 in vitro correlates with the clinical immune defect in chronic hemodialysis patients. Kidney Int. 1995;47:559–565. doi: 10.1038/ki.1995.70. [DOI] [PubMed] [Google Scholar]
- 2.Hunter CA, Ellis-Neyes LA, Slifer T, Kanaly S, Grunig G, Fort M, Rennick M, Araujo FG. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J. Immunol. 1997;158:3311. [PubMed] [Google Scholar]
- 3.Wu Y, Liu Z, Xiang Z, Zeng C, Chen Z, Ma X, Li L. Obesity-related glomerulopathy: Insights from gene expression profiles of the glomeruli derived from renal biopsy samples. Endocrinology. 2006;147:44–50. doi: 10.1210/en.2005-0641. [DOI] [PubMed] [Google Scholar]
- 4.Hakam AC, Hussain T. Renal angiotensin AT2 receptors are upregulated and mediate the candesartan induced natriuresis/dieresis in obese rats. Hypertension. 2005;45:270–275. doi: 10.1161/01.HYP.0000151622.47814.6f. [DOI] [PubMed] [Google Scholar]
- 5.Tallam LS, Jandhyala BS. Significance of exaggerated natriuresis after Angiotensin AT1 receptor blockade or angiotensin converting enzyme inhibition in obese Zucker rats. Clin Exp Pharmacol Physiol. 2001;28:433–440. doi: 10.1046/j.1440-1681.2001.03457.x. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Lemuz E, Murakami Y, Larrayoz-Roldan IM, Moughmian AJ, Pavel J, Nishioku T, Saavedra JM. Angiotensin II AT1 receptor blockade decreases lipopolysaccharide-induced inflammation in the rat adrenal gland. Endocrinology. 2008;149:5177–5188. doi: 10.1210/en.2008-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haugen EN, Croatt AL, Nath KA. Angiotensin II induces renal oxidant stress in vivo and heme oxygenase-1 in vivo and in vitro. Kidney Int. 2000;58:144–152. doi: 10.1046/j.1523-1755.2000.00150.x. [DOI] [PubMed] [Google Scholar]
- 8.Lin L, Philips WE, Manning RD., Jr. Intrarenal angiotensin II is associated with inflammation, renal damage and dysfunction in Dahl salt-sensitive hypertension. Am J Hypertens. 2009;3:306–314. doi: 10.1016/j.jash.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez-Presa M, Bustos C, Ortego M, Tunon J, Renedo G, Ruiz-Ortega M, Egido J. Angiotensin converting enzyme inhibition prevents arterial nuclear factor-kB activation, monocyte chemoattractant protein-1 protein expression and macrophage infiltration in a rabbit model of accelerated atherosclerosis. Circulation. 1997;95:1532–1541. doi: 10.1161/01.cir.95.6.1532. [DOI] [PubMed] [Google Scholar]
- 10.Kim JA, Berliner JA, Nadler JL. Angiotensin II increases monocyte binding to endothelial cells. Biochem Biophys Res Commun. 1996;226:862–868. doi: 10.1006/bbrc.1996.1441. [DOI] [PubMed] [Google Scholar]
- 11.Mene P, Fais S, Cinnoti GA, Pugliese F, Luttman W, Thierauch KH. Regulation of U-937 monocyte adhesion to human mesangial cells by cytokines and vasoactive agents. Nephrol Dial Transplant. 1995;10:481–489. doi: 10.1093/ndt/10.4.481. [DOI] [PubMed] [Google Scholar]
- 12.Guo G, Morrissey J, McCracken R, Tolley T, Liapis H, Klahr S. Contributions of angiotensin II and tumor-necrosis factor-α to the development of renal fibrosis. Am J Physiol Renal. 2001;280:F777–F785. doi: 10.1152/ajprenal.2001.280.5.F777. [DOI] [PubMed] [Google Scholar]
- 13.Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest. 1997;100:264–269. doi: 10.1172/JCI119531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carey RM, Jin X, Wang Z, Siragy HM. Nitric oxide: a physiological mediator of the type 2 (AT2) angiotensin receptor. Acta Physiol Scand. 2000;168:65–71. doi: 10.1046/j.1365-201x.2000.00660.x. [DOI] [PubMed] [Google Scholar]
- 15.Hakam AC, Hussain T. Angiotensin II AT2 receptors inhibit proximal tubular Na+K+ATPase activity via a NO/cGMP mediated pathway. Am J Renal Physiol. 2006;290:F1430–F1436. doi: 10.1152/ajprenal.00218.2005. [DOI] [PubMed] [Google Scholar]
- 16.Bedecs K, Elbaz N, Sutren M, Masson M, Susini C, Strosberg AD, Nahmias C. Angiotensin II type 2 receptors mediate inhibition of mitogen-activated protein kinase cascade and functional activation of SHP-1 tyrosine phosphatase. Biochem J. 1997;325:449–454. doi: 10.1042/bj3250449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horiuchi M, Hayashida W, Kambe T, Yamada T, Dzau VJ. Angiotensin II type 2 receptor dephosphorylates Bcl-2 by activating mitogen-activated protein kinase phosphatase-1 and induces apoptosis. J Biol Chem. 1997;272:19022–19026. doi: 10.1074/jbc.272.30.19022. [DOI] [PubMed] [Google Scholar]
- 18.Matavelli LC, Jiang H, Siragy HM. Angiotensin AT2 receptor stimulation inhibits early renal inflammation in renovascular hypertension. Hypertension. 2011;57:308–313. doi: 10.1161/HYPERTENSIONAHA.110.164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabuhi R, Ali Q, Asghar M, Al-Zamily NRH, Hussain T. Role of angiotensin II AT2 receptor in inflammation and oxidative stress: Opposing effects in lean and obese rats. Am J Renal Physiol. 2011;300:F700–706. doi: 10.1152/ajprenal.00616.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curato C, Slavic S, Dong J, Skorska A, Altarche-Xifro W, Miteva K, Kaschina E, Thiel A, Imboden H, Wang I, Steckelings U, Steinhoff G, Unger T, Li J. Identification of non-cytotoxic and IL-10 producing CD8+AT2R+ T-cell population in response to ischemic heart injury. J Immunol. 2010;185:6286–6293. doi: 10.4049/jimmunol.0903681. [DOI] [PubMed] [Google Scholar]
- 21.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension and progressive glomerular damage in Dahl rats. Kidney Int. 1984;26:137–143. doi: 10.1038/ki.1984.147. [DOI] [PubMed] [Google Scholar]
- 22.Wang F, Xia ZF, Chen XL, Jia YT, Wang YJ, Ma B. Angiotensin II type-1 receptor antagonist attenuates LPS-induced acute lung injury. Cytokine. 2009;48:246–253. doi: 10.1016/j.cyto.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Miyoshi M, Miyano K, Moriyama N, Taniguchi M, Watanabe T. Angiotensin type-1 receptor antagonist inhibits lipopolysaccharide-induced stimulation of rat microglial cells by suppressing nuclear factor κB and activator protein-1 activation. Eur J Neurosci. 2008;27:343–351. doi: 10.1111/j.1460-9568.2007.06014.x. [DOI] [PubMed] [Google Scholar]
- 24.Kornfeld P, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 25.Lavaud S, Michel O, Sassy-Prigent C, Heudes D, Bazin R, Bariety J, Chevalier J. Early influx of glomerular macrophages precedes glomerulosclerosis in the obese Zucker rat model. J Am Soc Nephrol. 1996;7:2604–2615. doi: 10.1681/ASN.V7122604. [DOI] [PubMed] [Google Scholar]
- 26.Yang N, Wu LL, Nikolic-Paterson DJ, Ng Y, Yang W, Mu W, Glibert RE, Cooper ME, Atkins RC, Lan HY. Local macrophage and myofibroblast proliferation in progressive renal injury in the rat remnant kidney. Nephrol Dial Transplant. 1998;13:1967–1974. doi: 10.1093/ndt/13.8.1967. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Tay Y, Harris DCH. Proximal tubule cells stimulated by lipopolysaccharide inhibit macrophage activation. Kidney Int. 2004;66:655–662. doi: 10.1111/j.1523-1755.2004.00786.x. [DOI] [PubMed] [Google Scholar]
- 28.Ozono R, Wang Z, Moore AF, Inagami T, Siragy HM, Carey RM. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension. 1997;30:1238–1246. doi: 10.1161/01.hyp.30.5.1238. [DOI] [PubMed] [Google Scholar]
- 29.Ali Q, Sabuhi R, Hussain T. High glucose upregulates angiotensin type 2 receptors via interferon regulatory factor-1 in proximal tubule epithelial cells. Mol Cell Biochem. 2010;344:65–71. doi: 10.1007/s11010-010-0529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rompe F, Artuc M, Hallberg A, Alterman M, Stroder K, Thone-Reineke C, Reichenback A, Schacherl J, Dahlof B, Bader M, Alenina N, Schwaninger M, Zuberbier T, Funke-Kaiser H, Schmidt C, Schunck W, Unger T, Steckelings UM. Direct angiotensin II type 2 receptor stimulation acts as anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor κB. Hypertension. 2010;55:924–931. doi: 10.1161/HYPERTENSIONAHA.109.147843. [DOI] [PubMed] [Google Scholar]
- 31.Abadir PM, Walton JD, Carey RM, Siragy HM. Angiotensin II type 2 receptors modulate inflammation through signal transducer and activator transcription proteins 3 phosphorylation and TNF-α production. J Interferon Cytokine Res. 2011;31:471–474. doi: 10.1089/jir.2010.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connelly L, Palacios-Callender M, Ameixa C, Moncada S, Hobbs AJ. Biphasic regulation of NFκB activity underlies the pro- and anti-inflammatory actions of nitric oxide. J Immunol. 2001;166:3873–3881. doi: 10.4049/jimmunol.166.6.3873. [DOI] [PubMed] [Google Scholar]
- 33.Peng H, Libby P, Liao JK. Induction and stabilization of IκBα by nitric oxide mediates inhibition of NFκB. J Biol Chem. 1995;270:14214–14219. doi: 10.1074/jbc.270.23.14214. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Zhang G, Feil R, Han J, Du X. Sequential activation of p38 and ERK pathways by cGMP-dependent protein kinase leading to activation of platelet integrin αIIbβ3. Blood. 2006;107:965–972. doi: 10.1182/blood-2005-03-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iliescu R, Chade AR. Progressive renal vascular proliferation and injury in obese Zucker rats. Microcirculation. 2010;17:250–258. doi: 10.1111/j.1549-8719.2010.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurtz TW, Morris RC, Pershadsingh HA. The Zucker fatty rat as a model of obesity and hypertension. Hypertension. 1989;13:896–901. doi: 10.1161/01.hyp.13.6.896. [DOI] [PubMed] [Google Scholar]
- 37.Kasiske B, O’Donnell MP, Cleary MP, Keane WF. Treatment of hyperlipidemia reduces glomerular injury in obese Zucker rats. Kidney Int. 1988;33:667–672. doi: 10.1038/ki.1988.51. [DOI] [PubMed] [Google Scholar]
- 38.Viswanathan M, de Oliveira AM, Wu RM, Chiueh CC, Saavedra JM. [125I]CGP42112 reveals a non-angiotensin II binding site in 1-methyl-4-phenylpyridine (MPP+)-induced brain injury. Cell Mol Neurobiol. 1994;14:99–104. doi: 10.1007/BF02088592. [DOI] [PubMed] [Google Scholar]
- 39.Macari D, Whitebread S, Cumin F, de Gasparo M, Levens N. Renal actions of the angiotensin AT2 receptor ligands CGP42112 and PD123319 after blockade of the renin angiotensin system. Eur J Pharmacol. 1994;259:27–36. doi: 10.1016/0014-2999(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 40.Coimbra TM, Janssen U, Grone HJ, Ostendorf T, Kunter U, Schmidt H, Brabant G, Floege J. Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int. 2000;57:167–182. doi: 10.1046/j.1523-1755.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- 41.Manigrasso MR, Ferroni P, Santilli F, Taraborelli T, Guagnano MT, Michetti N, Davi G. Association between circulating adiponectin and interleukin-10 levels in android obesity: effects of weight loss. J Clin Endocrinol Metab. 2005;90:5876–5879. doi: 10.1210/jc.2005-0281. [DOI] [PubMed] [Google Scholar]
- 42.Esposito K, Pontillo A, Giugliano F, Giugliano G, Marfella R, Nicoletti G, Giugliano D. Association of low interleukin-10 levels with the metabolic syndrome in obese women. J Clin Endocrinol Metab. 2003;88:1055–1058. doi: 10.1210/jc.2002-021437. [DOI] [PubMed] [Google Scholar]
- 43.Siddiqui A, Ali Q, Hussain T. Protective role of Angiotensin II subtype 2 receptor in blood pressure increase in obese Zucker rats. Hypertension. 2009;53:256–261. doi: 10.1161/HYPERTENSIONAHA.108.126086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang J, Chen C, Ren H, Han Y, He D, Zhou L, Hopfer U, Jose PA, Zeng C. Angiotensin AT2 receptor decreases AT1 receptor expression and function via NO/cGMP/Sp1in renal proximal tubule cells from Wistar-Kyoto rats. J Hypertens. 2012;30:1176–1184. doi: 10.1097/HJH.0b013e3283532099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1: AT1R Expression in HK-2 cells. Representative western blot for AT1R (~41 kDa) showing a detectable band with both 10 μg and 50 μg of loaded protein.
Fig. S2: AT2R Expression in HK-2 proximal tubule epithelial cells. Representative western blots for AT2R protein (approx. 40 and 45 kDa) top panel and for β–actin in the lower panel. Data are mean ± SEM (n = 3). * indicates p<0.001 vs control untreated HK-2 cells.
Fig. S3: (A) Interleukin-6 (IL-6) and (B) interleukin-10 (IL-10) concentration in the media collected from HK-2 cells stimulated by TNF-α (10 ng/ml) and/or C21 (1 umol/L). Data are mean ± SEM (n = 3). * indicates p<0.05 vs control untreated, # indicates p<0.05 vs TNF-α treated and $ indicates p<0.05 vs C21 treated HK-2 cells.
Fig. S4: Dose dependent increase in interleukin-10 (IL-10) production by HK-2 proximal tubule epithelial cells following treatment with AT2R agonist (C21; 0.1-10 μmol/L) for 24 hours. AT2R antagonist PD123319 (PD, 10 μmol/L) was able to block the C21-mediated increase in IL-10 concentration. Cytokine concentrations in media were measured by ELISA. Data are represented as mean ± SEM (n=5).
Fig. S5: Dose dependent increase in total nitrates in the media collected from HK-2 proximal tubule epithelial cells following treatment with AT2R agonist (C21; 0.1-10 μmol/L) for 24 hours. AT2R antagonist PD123319 (PD, 10 μmol/L) was able to block the C21-mediated increase in total nitrate production. Data are represented as mean ± SEM (n=5).
Fig. S6: Representative blots depicting the relative abundances of AT1R and AT2R in lean and obese Zucker rats.