Abstract
Objective
To examine whether persistent Metabolic syndrome (MetS) was associated with risk for type 2 diabetes in overweight Latino children.
Study design
73 participants (age 11.0±1.7 years) from a longitudinal study were classified as: NEVER (negative for MetS at all 3 visits); INTERMITTENT (positive for MetS at 1 or 2 visits); or PERSISTENT (positive for MetS at all 3 visits). Measures included DEXA, MRI, 2-h oral glucose tolerance test, and frequently sampled intravenous glucose tolerance test.
Results
The PERSISTENT group had a faster rate of fat mass gain than the NEVER group (20% vs. 15% gain of baseline value, p<0.05 for time*group interaction (time= visit)). Independent of body composition, the PERSISTENT group increased by 70% in insulin incremental area under the curve, and the other groups decreased (p<0.05 for time*group interaction). Despite no time*group interactions for insulin sensitivity, acute insulin response, or disposition index, the PERSISTENT maintained 43% lower insulin sensitivity (p<0.01) and by visit 2 had a 25% lower disposition index (p<0.05) compared with the NEVER group.
Conclusion
Participants with persistent MetS had accelerated fat gain, increasing insulin response to oral glucose, and lower insulin sensitivity and beta cell function, indicators of progressively greater risk for type 2 diabetes.
Keywords: longitudinal, beta cell function, insulin sensitivity, insulin resistance, insulin IAUC
Obesity and Latino ethnicity are two independent risk factors for the development of type 2 diabetes in youth. Even in childhood, there is a linear relationship between increased body fat and lower insulin sensitivity (1-4). Futhermore, independent of body composition, Latino children are more insulin resistant than white children (5). NHANES III data showed that the metabolic syndrome, a clustering of risk factors for diabetes and cardiovascular disease (6), was more common in Latino adolescents than in whites or blacks (7).
Thirty pecent of the Study of Latino Adolescents at Risk for Diabetes Project (SOLAR) cohort of overweight Latino youth had the metabolic syndrome (8). This cross-sectional analysis showed that insulin sensitivity was inversely associated with the number of features of the metabolic syndrome and that those with the metabolic syndrome (3+ features) had 62% lower insulin sensitivity than those with no features of the metabolic syndrome, independent of sex, age, sexual maturation, and body composition. However, we have not yet evaluated this relationship over time.
The overall objective of this study was to examine if the persistence of the metabolic syndrome was associated with changes in risk factors for type 2 diabetes within childhood in overweight Latino youth. The first aim was to identify how many children in the cohort consistently had the metabolic syndrome at three annual measurements. The second aim was to determine if participants with persistent metabolic syndrome had differences in insulin and glucose indices over time, independent of adiposity.
Methods
Participants were a subset of the University of Southern California SOLAR (Study of Latino Adolescents at Risk) for Diabetes Project, a longitudinal cohort study aimed to track the incidence of type 2 diabetes. Study inclusion criteria were: 1) Latino origin as defined by all four grandparents being Latino as determined by parental self-report; 2) family history of type 2 diabetes in at least one grandparent, parent, or sibling; 3) age 8-13; 4) body mass index (BMI) of at least the 85th percentile for age (9); and 5) absence of diabetes as confirmed by an oral glucose tolerance test (OGTT) (10). Participants (n=73) in the present analysis were selected because they had complete data for the metabolic syndrome parameters for each of the first 3 annual study visits. Mean age of the sample was 11.0 ±1.7 years at baseline. This sample (n=73) did not differ at baseline from the rest of the larger initial cohort (n=182) in key characteristics such as age, sex, Tanner stage, BMI, body composition, fasting glucose, 2 hour glucose, or insulin sensitivity (p>0.05 as assessed with independent t-tests and chi-square tests.) None of the participants were diabetic. This study was approved by the university Institutional Review Board. Written informed consent was obtained from parents and youth assent from participants.
Detailed methods for the longitudinal study have been previously published (8, 11, 12). Briefly, the design involves a set of yearly clinical assessments, consisting of an outpatient visit in which an OGTT is conducted, and an overnight, inpatient visit, in which a frequently sampled intravenous glucose tolerance test (FSIVGTT) is conducted.
Children fasted overnight and came to the General Clinical Research Center (GCRC) at 0800 h. Participants changed into hospital gowns and height and weight were recorded in triplicate to the nearest 0.1cm and 0.1 kg, respectively. BMI and BMI percentiles for age were calculated using the EpiInfo 2000 software, version 1.1, based upon established CDC normative curves (9). Sitting blood pressure was measured in triplicate (13). Tanner stage was coded to assess sexual maturation based on breast stage in girls and pubic hair in boys during a history and physical exam conducted by a licensed pediatric care provider (14). For the OGTT, subjects were given 1.75g of oral glucose solution /kg body weight (to a maximum of 75.0g). Blood was collected and assayed for glucose and insulin at −5min and 15, 30, 45, 60, and 120 min relative to glucose ingestion. Impaired glucose tolerance was defined as a 2-h post challenge plasma glucose value of at least 140 and less than 200mg/dl (10). Two-hour insulin and glucose area under the curve (AUC) and incremental area under the curve (IAUC) were calculated from the OGTT data, in mg/min/dl for glucose and μU/min/ml for insulin. Glucose and insulin AUC are calculated as the sum of the area of each time segment by insulin or glucose concentration, and IAUC as the sum of the same area adjusted for the starting point.
Participants were admitted to the GCRC for the inpatient visit in the afternoon and fasted from 2000 h until testing the following morning, which began at 0730 h. Sitting blood pressure was again taken in triplicate, and the values from the two visits were averaged. A flexible iv catheter was placed in each arm and the FSIVGTT was conducted. At time zero, subjects were given a 0.3 g/kg body weight dose of glucose (25% dextrose) with sampling at 2, 4, 8, 19, 22, 30, 40, 50, 70, 100, and 180 minutes. At 20 minutes, a 0.02 U /kg body weight dose of Humulin® R insulin (regular insulin for human participants; Eli Lilly, Indianapolis, USA) was injected. In order to determine insulin sensitivity (SI) and the acute insulin response to glucose (AIR), values for glucose and insulin were entered into the MINMOD MILLENIUM 2002 program (Version 5.16, Richard N. Bergman). The disposition index (DI), an index of the compensatory adaptation to insulin resistance, was calculated as the product of SI and AIR, and is used to approximate beta cell function. Fasting blood samples were also measured for triglycerides, and total and HDL cholesterol using the Vitros chemistry DT slides (Johnson and Johnson Clinical Diagnostics Inc., Rochester, NY).
After the FSIVGTT, body composition was measured by a whole-body dual-energy x-ray absorptiometry (DEXA) scan by a certified Radiological Technologist using a Hologic QDR 4500W (Hologic, Bedford, MA.) A urine pregnancy test was given to all female participants prior to DEXA. In addition, waist circumference, measured at the umbilicus, was recorded to the nearest 0.1cm. Central fat distribution was measured by magnetic resonance imaging (MRI) at the LAC/USC Imaging Science Center using a GE 1.5 Signa LX-Ecospeed with a GE 1.5 Tesla magnet and a single slice at the level of the umbilicus. This procedure measures intra-abdominal adipose tissue (IAAT) and subcutaneous abdominal adipose tissue (SAAT).
No standard definition of the metabolic syndrome has been agreed upon for children/adolescents (15, 16). For this analysis, the metabolic syndrome was categorized using a definition we have previously proposed (8) which applies pediatric cutoffs to the Adult Treatment Panel III definition (17). The metabolic syndrome was defined as having ≥3 of the following: 1) abdominal obesity (waist circumference ≥90th percentile for age, sex, and Hispanic ethnicity from NHANES III data) (18), hypertriglyceridemia (triglycerides ≥90th percentile of age and sex) (19), low HDL cholesterol (HDL cholesterol ≤ 10th percentile for age and sex) (19), elevated blood pressure (systolic or diastolic blood pressure >90th percentile adjusted for height, age, and sex) (13), and impaired glucose tolerance, as described above.
Participants were classified in 3 groups: NEVER (negative for metabolic syndrome at all 3 annual visits); INTERMITTENT (positive for metabolic syndrome at 1 or 2 annual visits); and PERSISTENT (positive for metabolic syndrome at all 3 annual visits).
Baseline characteristics of the 3 groups were compared using chi-square tests and analysis of variance (ANOVA) with Bonferroni corrections. All participants had complete data for the 5 features of the metabolic syndrome at all 3 time points. However, subjects were still included if they were missing data for MRI, DEXA, IAUC from the OGTT, or IVGTT (SI, AIR, or DI) measurements. At baseline, 1 subject had missing data for SI, DI, and AIR and 6 were missing MRI. Two year changes in adiposity as well as insulin dynamics, measured by SI, AIR, and DI, were analyzed with repeated measures analysis of covariance (ANCOVA). For the ANCOVA analyses, the following are the numbers for missing data: 2 DEXA, 9 SAAT, 10 IAAT, 8 glucose IAUC, 4 insulin IAUC, and 5 SI, DI, and AIR. The class variable was metabolic syndrome group and the time variable was annual visit number (1-3). For ANCOVA, the following covariates were included in all models: sex, baseline age and baseline Tanner stage. Baseline lean tissue mass was also controlled for when fat mass, SAAT, or IAAT was the outcome. In the IAAT models, baseline SAAT was controlled for, and vice-versa. In models where insulin and glucose indices were evaluated, baseline body composition (fat mass and lean tissue mass) were controlled for. Models were also run with the inclusion of Tanner stage and body composition at all time points but these covariates were not significant, so models including baseline values were used. For repeated measures ANCOVA, Mauchly test of sphericity was used to assess the form of the common covariance matrix. When the sphericity assumption was not met, the Huynh-Feldt correction was used. Data were analyzed with SPSS version 13.0 (SPSS Inc, Chicago, IL), and type 1 error was set at α<0.05.
Results
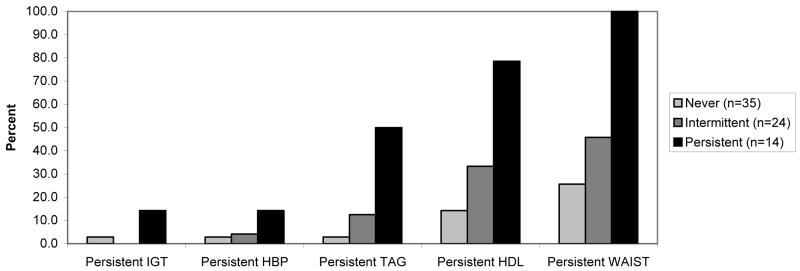
Of the 73 children, 35 (48%) did not have the metabolic syndrome at any of the 3 annual visits and were classified into the NEVER group. Twenty four (33%) of the participants were in the INTERMITTENT group, as they had the metabolic syndrome at one or two visits. Fourteen children (19%) had the metabolic syndrome at all three visits and were classified as PERSISTENT. The persistence of each individual feature, displayed by metabolic syndrome group, is shown in Figure 1 (available at www.jpeds.com). The most commonly persistent features were high waist circumference, followed by low HDL cholesterol and high triglycerides. The percentage of participants displaying persistent metabolic syndrome features was incremental by metabolic syndrome group, with the lowest percentage in the NEVER group, followed by the INTERMITTENT group, and the PERSISTENT group. The number of features met by each of the metabolic syndrome groups was also incremental, with the NEVER group having an average of 1.02 features, the INTERMITTENT group having an average of 2.18 features, and the PERSISTENT group having an average of 3.48 features (data not shown).
Figure 1.
Persistence of each metabolic syndrome feature by metabolic syndrome group.
Never =negative for metabolic syndrome at all 3 annual visits; Intermittent =positive for metabolic syndrome at 1 or 2 visits; Persistent =positive for metabolic syndrome at all 3 visits. Repeated measures analysis of covariance used to compare groups. Predicted values adjusted for sex and baseline age, Tanner stage, and total lean tissue mass. Figures 2b-d also adjusted for total fat mass.
Baseline unadjusted descriptive characteristics of the participants by metabolic syndrome group are shown in Table I. Age did not differ across groups. The NEVER group had fewer male participants (p<0.05) and lower BMI than the other groups (p<0.05). In addition, the NEVER group had lower fat mass (p<0.05) and lean mass (p<0.01) when compared with the INTERMITTENT group. There was an overall group difference in SAAT p<0.05, but no differences in IAAT (p>0.05). Participants in the PERSISTENT group had a lower mean Tanner stage than those in the other groups, indicating that they were less sexually mature (p<0.05). Comparisons of the features of the metabolic syndrome at baseline are also shown in Table I. As expected by group definitions, the NEVER group had lower waist circumference, blood pressure, and triglycerides as well as higher HDL cholesterol than the other groups (p<0.05), but 2 hour glucose values did not differ by group.
Table 1.
Baseline unadjusted descriptive characteristics and individual metabolic syndrome features by metabolic syndrome group in overweight Latino children
| Variable | Pearson Chi-Square | Never (N) (n=35) | Intermittent (I) (n=24) | Persistent (P) (n=14) | |||
|---|---|---|---|---|---|---|---|
| Male sex (%) | * | 31.40% | 62.50% | 64.30% | |||
| Omnibus Test | Significant Comparisons | ||||||
| N vs. I | N vs. P | I vs. P | |||||
| Tanner stage | * | 2.43 ± 1.20 | 2.54 ± 1.50 | 1.43 ± 1.09 | * | * | |
| Age (years) | 11.0 ± 1.7 | 11.6 ± 1.8 | 10.4 ± 1.4 | ||||
| BMI (kg/m2) | ** | 25.7 ± 4.5 | 29.6± 6.1 | 29.7 ± 5.0 | * | * | |
| BMI percentile | *** | 95.2 ± 3.9 | 97.8 ± 2.1 | 98.7 ± 1.0 | ** | ** | |
| Total fat mass (kg) | * | 20.5 ± 8.1 | 27.1 ± 11.3 | 26.8 ± 10.9 | * | ||
| Total lean tissue mass (kg) | ** | 33.0 ± 7.8 | 41.5 ± 11.9 | 35.8 ± 9.6 | ** | ||
| Waist circumference (cm) | ** | 81.4 ± 11.5 | 91.1 ± 12.3 | 91.0 ± 14.6 | * | ||
| SAAT (cm2) | * | 280.4 ± 111.2 (n=31) | 347.2 ± 131.3 (n=23) | 372.1 ± 144.6 (n=13) | |||
| IAAT (cm2) | 44.7 ± 20.0 (n=31) | 46.2 ± 18.7 (n=23) | 54.8 ± 19.3 (n=13) | ||||
| Systolic blood pressure (mm Hg) | *** | 105.1 ± 9.1 | 110.6 ± 8.5 | 116.5 ± 6.4 | * | *** | |
| Diastolic blood pressure (mm Hg) | ** | 60.8 ± 4.6 | 61.9 ± 5.2 | 65.9 ± 3.7 | ** | * | |
| HDL cholesterol (mg/dl) | *** | 44.6 ± 10.5 | 36.2 ± 6.6 | 32.6 ± 4.8 | ** | *** | |
| Triglycerides (mg/dl) | *** | 90.6 ± 39.9 | 126.5 ± 43.9 | 143.0 ± 53.9 | ** | ** | |
| 2 hour glucose (mg/dl) | 124.0 ± 17.6 | 123.0 ± 16.3 | 131.1 ± 15.2 | ||||
Never=negative for metabolic syndrome at all 3 annual visits, Intermittent=positive for metabolic syndrome at 1 or 2 visits. Persistent = positive for metabolic syndrome at all 3 visits. SAAT=subcutaneous adipose tissue, IAAT=intra-abdominal adipose tissue.
Chi-square test used for sex and Tanner stage comparisons and data are percentages or medians. ANOVA performed to compare means with Bonferroni corrections for multiple comparisons and data are means ± standard deviations.
P<0.05,
P<0.01,
P<0.001
Baseline unadjusted insulin and glucose related indices of the three groups are found in Table II. The INTERMITTENT group had higher fasting insulin than the NEVER group (p<0.01), but fasting glucose, glucose IAUC, or insulin IAUC did not differ by group. SI was higher in those who NEVER had the metabolic syndrome compared with the other 2 groups (p<0.05) but AIR or DI did not differ by group.
Table 2. Baseline unadjusted indices of insulin and glucose by metabolic syndrome group in overweight Latino children.
| Significant Comparisons | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Ombibus Test | Never (N) (n=35) | Intermittent (I) (n=24) | Persistent (P) (n=14) | N vs. I | N vs. P | I vs.P |
| Fasting glucose (mg/dl) | 89.9 ± 6.9 | 91.5 ± 6.3 | 93.6 ± 6.3 | ||||
| 2 hour glucose (mg/dl) | 124.0 ± 17.6 | 123.0 ± 16.3 | 131.1 ± 15.2 | ||||
| Glucose IAUC (mg/min/dl) | 87.4 ± 36.1 | 74.5 ± 31.9 | 94.5 ± 45.3 | ||||
| Fasting insulin (μU/ml) | ** | 11.8 ± 6.7 | 20.9 ± 11.8 | 18.0 ± 10.4 | ** | ||
| 2 hour insulin (μU/ml) | 120.0 ±94.2 | 164.8 ± 142.4 | 157.4 ± 110.8 | ||||
| Insulin IAUC (μU/min/ml) | 237.7 ± 144.8 | 285.1 ± 226.5 | 299.8 ± 132.4 | ||||
| Insulin sensitivity (X10-4 min -1/ μU/ml) | ** | 2.7 ± 1.5 (n=34) | 1.7 ± 1.0 | 1.6 ± 0.6 | ** | * | |
| Acute insulin response (μU/ml X 10 min) | 1357± 1020 (n=34) | 2192 ± 1709 | 1736 ± 941 | ||||
| Disposition index (X10-4 min -1) | 2660± 1341 (n=34) | 2597 ± 1067 | 2387 ± 915 | ||||
Never=negative for metabolic syndrome at all 3 annual visits, Intermittent=positive for metabolic syndrome at 1 or 2 visits. Persistent = positive for metabolic syndrome at all 3 visits. IAUC=incremental area under the curve, SI=insulin sensitivity, AIR=acute insulin response, DI=disposition index (a measure of beta cell function).
ANOVA performed to compare means with Bonferroni corrections for multiple comparisons and data are means ± standard deviations.
P<0.05,
P<0.01,
P<0.001
Results from the repeated measures ANCOVA are shown in Table III. Although overall BMI percentile did not change significantly over time, the NEVER group maintained a lower adjusted BMI percentile as compared with the other groups (p<0.05). The PERSISTENT group gained fat mass at a faster rate than the NEVER group (Figure 2, A, 20% gain of baseline value by visit 3 vs. a 15% gain, p=0.024 for time*group interaction). The PERSISTENT group also maintained a significantly higher level of SAAT than the NEVER group (p=0.048), but this difference was stable over time. IAAT was not significantly affected by time, group status, or an interaction of time*group status.
Table 3. Repeated measures analysis of variance for adiposity measures and insulin/glucose indices by metabolic syndrome group in overweight Latino children.
| Adjusted values | Time | Metabolic syndrome group | Time*Group | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Omnibus test | Group contrasts | ||||||||
| Baseline (Visit 1) | 1 year follow-up (Visit 2) | 2 year follow-up (Visit 3) | N vs. I | N vs. P | I vs. P | ||||
| BMI (kg/m2) a | 0.642 | 0.001 | * | *** | 0.445 | ||||
| Never (n=35) | 25.7 ± 3.3 | 26.8 ± 3.2 | 28.8 ± 3.6 | ||||||
| Intermittent (n=24) | 29.6 ± 3.4 | 31.3 ± 3.5 | 31.5 ± 3.6 | ||||||
| Persistent (n=14) | 29.7 ± 2.8 | 32.0 ± 2.8 | 33.2 ± 2.9 | ||||||
| BMI percentile a | 0.312 | <0.001 | ** | *** | 0.481 | ||||
| Never (n=35) | 95.2 ± 0.9 | 95.2 ± 0.7 | 94.0 ± 0.9 | ||||||
| Intermittent (n=24) | 97.8 ± 0.9 | 98.0 ± 0.8 | 97.1 ± 1.0 | ||||||
| Persistent (n=14) | 98.7 ± 0.9 | 99.0 ± 0.7 | 98.9 ± 0.8 | ||||||
| Fat mass(kg) a | 0.066 | 0.106 | * | 0.024 | |||||
| Never (n=35) | 20.5 ± 6.7 | 22.5 ± 6.2 | 23.6 ± 5.7 | ||||||
| Intermittent (n=24) | 27.1 ± 9.2 | 30.5 ± 8.6 | 30.6 ± 7.5 | ||||||
| Persistent (n=12) | 27.2 ± 8.5 | 30.7 ± 7.6 | 32.8 ± 6.7 | ||||||
| SAAT (cm2) a | 0.059 | 0.138 | * | 0.542 | |||||
| Never (n=31) | 280.4 ± 90.2 | 307.2 ± 91.9 | 320.8 ± 85.3 | ||||||
| Intermittent (n=21) | 356.1 ± 118.5 | 416.1 ± 117.4 | 412.4 ± 105.0 | ||||||
| Persistent (n=12) | 370.1 ± 113.9 | 422.1 ± 114.1 | 442.6± 101.5 | ||||||
| IAAT (cm2) | 0.053 | 0.342 | 0.268 | ||||||
| Never (n=31) | 44.7 ± 9.7 | 36.4 ± 7.2 | 36.4 ± 11.0 | ||||||
| Intermittent (n=21) | 46.5 ± 11.2 | 48.9 ± 8.4 | 55.7 ± 13.0 | ||||||
| Persistent (n= 11) | 51.6 ± 11.3 | 47.9 ± 6.9 | 55.2 ± 11.5 | ||||||
| Fasting glucose (mg/dl) | 0.408 | 0.012 | ** | * | 0.481 | ||||
| Never (n=35) | 89.9 ± 2.9 | 89.7 ± 0.7 | 91.3 ± 1.5 | ||||||
| Intermittent (n=24) | 91.5 ± 3.1 | 92.3 ± 0.9 | 93.2 ± 2.5 | ||||||
| Persistent (n=14) | 93.6 ± 2.5 | 96.2 ± 0.6 | 98.6 ± 1.7 | ||||||
| 2-hour glucose (mg/dl) | 0.045 | <0.001 | * | *** | * | 0.251 | |||
| Never (n=35) | 124 ± 4.8 | 122.7 ± 4.3 | 116.5 ± 5.4 | ||||||
| Intermittent (n=24) | 123.0 ± 6.1 | 129.3 ± 6.7 | 127.2 ± 5.5 | ||||||
| Persistent (n=14) | 131.1 ± 6.1 | 140.0 ± 4.9 | 136.8 ± 4.6 | ||||||
| Glucose IAUC (mg/min/dl) | 0.461 | 0.005 | ** | * | 0.238 | ||||
| Never (n=33) | 85.7 ± 13.8 | 81.7 ± 12.0 | 69.9 ± 9.2 | ||||||
| Intermittent (n=20) | 72.4 ± 20.0 | 81.7 ± 16.2 | 79.6 ± 12.5 | ||||||
| Persistent (n=12) | 94.6 ± 19.2 | 102.3 ± 18.0 | 103.0 ± 13.9 | ||||||
| Fasting insulin (μU/ml) | 0.031 | 0.146 | 0.196 | ||||||
| Never (n=34) | 11.5 ± 3.5 | 15.2 ± 3.1 | 16.1 ± 5.1 | ||||||
| Intermittent (n=23) | 20.1 ± 5.1 | 20.3 ± 4.5 | 16.8 ± 5.8 | ||||||
| Persistent (n=14) | 18.0 ± 5.0 | 25.6 ± 3.7 | 25.7 ± 4.5 | ||||||
| 2-hour insulin (μU/ml) | 0.828 | 0.007 | ** | * | 0.029 | ||||
| Never (n=34) | 125.1 ± 40.9 | 159.5 ± 44.7 | 93.8 ± 44.5 | ||||||
| Intermittent (n=23) | 171.0 ± 48.5 | 171.6 ± 57.6 | 157.4 ± 52.3 | ||||||
| Persistent (n=14) | 157.4 ± 40.2 | 286.9 ± 40.6 | 281.2 ± 43.0 | ||||||
| Insulin IAUC a (μU/min/ml) | 0.025 | 0.025 | * | * | 0.012 | ||||
| Never (n=34) | 234.3 ± 68.1 | 293.8 ± 69.1 | 210.6 ± 83.8 | ||||||
| Intermittent (n=22) | 289.9 ± 92.0 | 311.2 ± 94.4 | 277.7 ± 104.8 | ||||||
| Persistent (n=13) | 302.6 ± 74.2 | 449.4 ± 76.1 | 516.0 ± 98.9 | ||||||
| SI a (X10-4 min -1/ μU/ml) | 0.001 | 0.019 | ** | 0.872 | |||||
| Never (n=32) | 2.78 ± 0.7 | 2.04 ± 0.5 | 1.94 ± 0.3 | ||||||
| Intermittent (n=23) | 1.71 ± 0.9 | 1.43 ± 0.6 | 1.37 ± 0.4 | ||||||
| Persistent (n=13) | 1.7 ± 0.8 | 1.11 ± 0.5 | 1.06 ± 0.3 | ||||||
| AIR (μU/ml X 10 min) | 0.106 | 0.506 | 0.302 | ||||||
| Never (n=32) | 1336.4 ± 466.5 | 1493.5 ± 475.8 | 1519.0 ± 296.8 | ||||||
| Intermittent (n=23) | 2211.3 ± 684.7 | 2132.5 ± 634.7 | 1882.3 ± 381.0 | ||||||
| Persistent (n=13) | 1568.0 ± 740.1 | 1973.1 ± 649.2 | 1930.2 ± 410.7 | ||||||
| DI (X10-4 min -1) | 0.419 | 0.044 | * | * | 0.482 | ||||
| Never (n=32) | 2606.7 ± 491.3 | 2454.5 ± 562.5 | 2319.0 ± 410.8 | ||||||
| Intermittent (n=23) | 2607.6 ± 634.9 | 2313.9 ± 714.3 | 2066.8 ± 435.8 | ||||||
| Persistent (n=13) | 2368.3 ± 404.7 | 1845.3 ± 361.1 | 1669.6 ± 233.1 | ||||||
Never=negative for metabolic syndrome at all 3 annual visits, Intermittent=positive for metabolic syndrome at 1 or 2 visits. Persistent = positive for metabolic syndrome at all 3 visits. SAAT=subcutaneous adipose tissue, IAAT=intra-abdominal adipose tissue, IAUC=incremental area under the curve, SI=insulin sensitivity, AIR=acute insulin response, DI=disposition index (a measure of beta cell function).
Repeated measures ANOVA used to compare changes in insulin and glucose dynamics over visits 1-3 and data are means ± standard deviations.
P<0.05,
P<0.01,
P<0.001
All analyses are adjusted for sex and baseline age, and Tanner stage. Total lean tissue mass was also controlled for in the total fat mass model, and total lean and total fat were controlled for in all insulin and glucose indices models.
Sphericity assumption violated and huynh-feldt correction used.
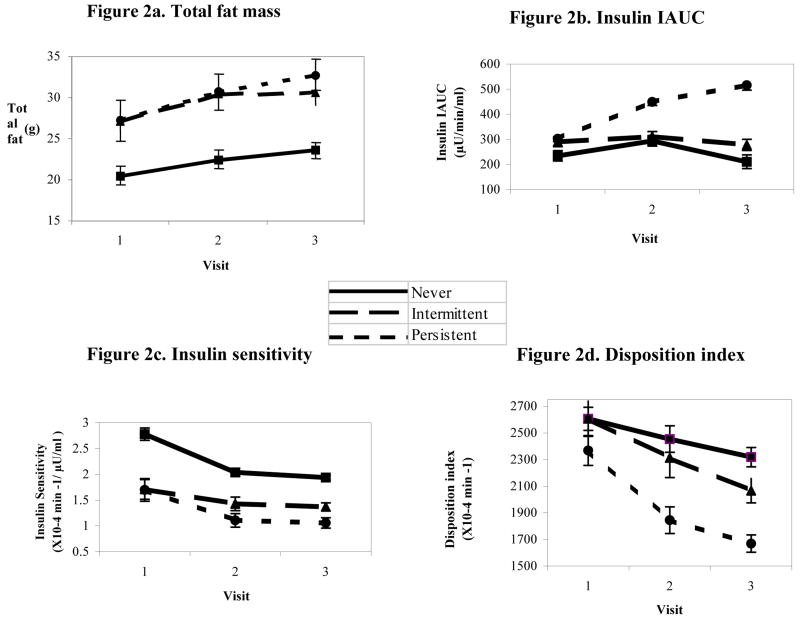
Figure 2.
Changes in fat mass and insulin/glucose indices over 3 annual visits by metabolic syndrome group in overweight Latino children
Figure 2, A: Total fat mass (n=71). Effect of time: p=0.066; Differences by group: omnibus p=0.106, N vs P p=0.039; Interaction for time by group p=0.024. Figure 2, B: Insulin IAUC (n=69). Effect of time: p=0.025; Differences by group: omnibus p=0.025, N vs. P p=0.012, I vs. P p=0.018; Interaction for time by group p=0.012. Figure 2, C: Insulin sensitivity (n=68). Effect of time: p=0.001; Differences by group: omnibus p=0.019, N vs. P p=0.006, N vs. I p=0.073; Interaction for time by group p=0.872. Figure 2, D: Disposition index (n=68) Effect of time: p=0.419; Differences by group: omnibus p=0.044, N vs. P p=0.020, I vs P p=0.024; Interaction for time by group: p=0.482.
Repeated measures ANCOVA results also revealed longitudinal differences in indices related to insulin and glucose, independent of covariates including body composition. The PERSISTENT group maintained higher fasting glucose, 2 hour glucose, and glucose IAUC levels than the other two groups (p<0.05), but these differences were stable over time. However, changes in 2 hour insulin and insulin IAUC over time were significantly associated with metabolic syndrome group; the PERSISTENT group increased by over 70% in both, but the other groups had an overall decrease (Figure 2, B, p<0.05 for time*group interaction). In addition, although all participants, regardless of group, declined in adjusted SI over time (p=0.001), the PERSISTENT group remained 43% less insulin sensitive, on average, as compared with those in the NEVER group (Figure 2, C, p=0.006). Furthermore, despite no significant differences in baseline DI, by visit 2, the PERSISTENT group had a 25% lower adjusted DI than the NEVER group, and this difference was maintained through visit 3 (Figure 2, D, p=0.02). However, the rates of change did not differ by group for DI or for AIR (time*group interaction>0.05) and there were no group differences in AIR.
Discussion
This study examined the persistence of the metabolic syndrome over time within childhood, and focused specifically on associations with risk for type 2 diabetes in overweight Latino youth. In a study by Goodman et al, 1,098 adolescents (52% white, 47% black, and 2% Latino) were evaluated for the metabolic syndrome at baseline (average age 15 years) and at followup three years later (20). The authors conclude that clinical categorization of the metabolic syndrome was not stable and that the syndrome has limited clinical utility for adolescents (20). However, associations with insulin sensitivity and beta cell function were not evaluated. In another study, by Weiss et al, the metabolic sydrome was also assessed at two time points, an average of 22 months apart, in a sub-sample of 77 youth who were ages 4-20 years at baseline (21). Though demographic information is not given for the sub-sample, the larger study sample is 27% Latino. The authors found that 71% of the subjects who had the metabolic syndrome at the first assessment also had it at the second assessment. In addition, eight subjects who had impaired glucose tolerance and the metabolic syndrome at the first assessment had developed type 2 diabetes by the second assessment.
In addition to the two studies which evaluated the persistence of the metabolic syndrome, several other studies have evaluated either predictors of the metabolic syndrome in childhood or associations between childhood metabolic syndrome and risk for type 2 diabetes later in life. In a study of 154 white girls who were measured for adiposity at ages 5, 7, 9, 11, and 13 years, as well as measured for the metabolic syndrome at age 13 only, Ventura et al found that increases in fat mass and BMI across childhood were predictive of metabolic syndrome risk at age 13 (22). In a study of 1,604 American Indians, Franks et al found that a composite score of metabolic syndrome features in 5-19 year olds was a predictor of the development of type 2 diabetes (23). Morrison et al also found that in a sample of youth (72% white and 28% black, ages 5-19 years at baseline), the metabolic syndrome in childhood was a significant predictor of type 2 diabetes 25 to 30 years later (24). As far as we know, our study is the first to show that progressive risk for type 2 diabetes is evident with persistent metabolic syndrome over just three consecutive annual measurements within childhood.
The underlying pathophysiology for the progressive risk for type 2 diabetes in the PERSISTENT group, particularly as compared with the NEVER group, is comprised of increasing adiposity, consistently lower SI, as well as a lesser ability of the beta cells to compensate by increasing insulin secretion, i.e. DI, which was lower by visit 2 and stayed consistently lower through visit 3. Other research has shown that insulin resistance and impaired insulin secretion are independent predictors of the development of type 2 diabetes in adult Pima Indians and Latinos (25-27). Though all of the participants in our analysis showed an overall decline in SI across the 3 annual visits, the PERSISTENT group showed a dramatically accelerated increase in insulin response to oral glucose, which supports the conclusion that they are becoming progressively more insulin resistant than the other two groups.
It is important to note that all of the participants in our analysis, regardless of their metabolic syndrome group status, have lower SI than normal weight children of their same age. To put our results into context, in a previous analysis with a multi-ethnic group of children (mean age 9.6 yrs), Goran et al showed an inverse relationship between fat mass and SI, independent of family history of type 2 diabetes(28). This group of children, which had an average BMI of around 20, had an average SI of around 6. In comparison, the NEVER participants in the current analysis had an average baseline SI of 2.7, followed by an average of 1.7 and 1.6 in the INTERMITTENT and NEVER groups. Therefore, though all of our participants are relatively insulin resistant, the groups with more metabolic syndrome features are more dramatically so. It is also worthwhile to note that at baseline the INTERMITTENT and PERSISTENT groups were fairly comparable in terms of adiposity and insulin/glucose indices, including SI. However, by following these groups across 3 visits, we found that the PERSISTENT group emerged as the group which showed stronger associations with progressive diabetes risk, even when compared with the INTERMITTENT group.
Considering that the PERSISTENT group was less sexually mature, as indicated by Tanner staging, at baseline compared with the other two groups, one important question that arises is whether the PERSISTENT group progressed through puberty at a different rate than the other groups, which could have influenced their metabolic health. To answer this question, we assessed the changes in Tanner stage of the 3 groups by repeated measures ANOVA (data not shown). We found that although the PERSISTENT group started at a lower Tanner stage of 1.4, as compared with a 2.4 and 2.5 in the other groups, all groups progressed in Tanner stage at the same rates, i.e we did not see a time*group interaction in Tanner stage over time. However, we did include Tanner stage as a covariate in all of the adiposity and glucose/insulin models presented in this paper, in order to make sure that our results are not driven by differences in pubertal development.
Our findings have several important implications for clinical screening of overweight youth for associated metabolic co-morbidities. Although current consensus statements include screening for both type 2 diabetes and features of metabolic syndrome (6, 36), the value of repetitive assessment for metabolic syndrome in youth has been unclear. We show that assessing overweight Latino children in our sample for the metabolic syndrome on a yearly basis is useful in identifying those at particularly heightened risk for type 2 diabetes. Yearly metabolic syndrome assessment is achievable and relatively inexpensive, whereas conducting a FSIVGTT, which takes 3 hours to complete and requires the injection of glucose and insulin, is not a practical screening tool. Logistical consideration needs to be given to which definition of the metabolic syndrome is used, however. For example, in a clinic setting, it would be easier to measure fasting blood glucose rather than 2 hour glucose from an OGTT, as we used in this study. To address this concern, our group conducted a cross-sectional comparison in our cohort using three published pediatric definitions for the metabolic syndrome, including one definition (7) that used fasting glucose rather than 2 hour glucose, and found that regardless of which was used (7, 8, 21), the inverse relationship between metabolic syndrome features and SI was maintained (15).
A limitation that should be considered for the present study is the relatively small overall sample size and the uneven sample sizes between metabolic syndrome groups, which may preclude additional findings. In addition, the generalizability of these findings is limited to Latino youth in a similar age range (∼8-13 years) with a family history of diabetes. However, these limitations are offset by the strength of the longitudinal study design, along with the well-defined study population and the use of rigorous measures of metabolic status, such as DEXA for adiposity and FSIVGTT for SI.
Acknowledgments
We would like to thank study coordinators Christina Ayala, MPH, and Quintilia Avila, MPA, and the SOLAR team for the facilitation of the study visits as well as the nursing staff at the GCRC. In addition, we are grateful for our study participants and their families for their involvement.
This work was supported by National Institutes of Health (Grant R01 DK 59211), GCRC National Center of Research Resources (Grant MO1 RR 00043), and NCI Centers for Transdisciplinary Research on Energetics and Cancer (TREC) (Grant U54 CA 116848).
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. Prevalence and determinants of insulin resistance among U.S. adolescents: a population-based study. Diabetes Care. 2006;29:2427–32. doi: 10.2337/dc06-0709. [DOI] [PubMed] [Google Scholar]
- 2.Cruz ML, Bergman RN, Goran MI. Unique effect of visceral fat on insulin sensitivity in obese Hispanic children with a family history of type 2 diabetes. Diabetes Care. 2002;25:1631–6. doi: 10.2337/diacare.25.9.1631. [DOI] [PubMed] [Google Scholar]
- 3.Goran MI, Bergman RN, Gower BA. Influence of total vs. visceral fat on insulin action and secretion in African American and white children. Obes Res. 2001;9:423–31. doi: 10.1038/oby.2001.56. [DOI] [PubMed] [Google Scholar]
- 4.Goran MI, Ball GD, Cruz ML. Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. J Clin Endocrinol Metab. 2003;88:1417–27. doi: 10.1210/jc.2002-021442. [DOI] [PubMed] [Google Scholar]
- 5.Goran MI, Bergman RN, Cruz ML, Watanabe R. Insulin resistance and associated compensatory responses in African-American and Hispanic children. Diabetes Care. 2002;25:2184–90. doi: 10.2337/diacare.25.12.2184. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM. A constellation of complications: the metabolic syndrome. Clin Cornerstone. 2005;7:36–45. doi: 10.1016/s1098-3597(05)80066-3. [DOI] [PubMed] [Google Scholar]
- 7.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Pediatr Adolesc Med. 2003;157:821–7. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 8.Cruz ML, Weigensberg MJ, Huang TT, Ball G, Shaibi GQ, Goran MI. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab. 2004;89:108–13. doi: 10.1210/jc.2003-031188. [DOI] [PubMed] [Google Scholar]
- 9.National Center for Health Statistics CDC. Growth Charts: United States. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2000. [Google Scholar]
- 10.American Diabetes Association. clinical practice recommendations 2002. Diabetes Care. 2002;25(1):S1–147. doi: 10.2337/diacare.25.2007.s1. [DOI] [PubMed] [Google Scholar]
- 11.Goran MI, Bergman RN, Avila Q, Watkins M, Ball GD, Shaibi GQ, et al. Impaired glucose tolerance and reduced beta-cell function in overweight Latino children with a positive family history for type 2 diabetes. J Clin Endocrinol Metab. 2004;89:207–12. doi: 10.1210/jc.2003-031402. [DOI] [PubMed] [Google Scholar]
- 12.Weigensberg MJ, Ball GD, Shaibi GQ, Cruz ML, Goran MI. Decreased beta-cell function in overweight Latino children with impaired fasting glucose. Diabetes Care. 2005;28:2519–24. doi: 10.2337/diacare.28.10.2519. [DOI] [PubMed] [Google Scholar]
- 13.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76. [PubMed] [Google Scholar]
- 14.Tanner JM. Growth and maturation during adolescence. Nutr Rev. 1981;39:43–55. doi: 10.1111/j.1753-4887.1981.tb06734.x. [DOI] [PubMed] [Google Scholar]
- 15.Shaibi GQ, Goran MI. Examining metabolic syndrome definitions in overweight Hispanic youth: a focus on insulin resistance. J Pediatr. 2008;152:171–6. doi: 10.1016/j.jpeds.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones KL. The dilemma of the metabolic syndrome in children and adolescents: disease or distraction? Pediatr Diabetes. 2006;7:311–21. doi: 10.1111/j.1399-5448.2006.00212.x. [DOI] [PubMed] [Google Scholar]
- 17.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439–44. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 19.Hickman TB, Briefel RR, Carroll MD, Rifkind BM, Cleeman JI, Maurer KR, et al. Distributions and trends of serum lipid levels among United States children and adolescents ages 4-19 years: data from the Third National Health and Nutrition Examination Survey. Prev Med. 1998;27:879–90. doi: 10.1006/pmed.1998.0376. [DOI] [PubMed] [Google Scholar]
- 20.Goodman E, Daniels SR, Meigs JB, Dolan LM. Instability in the diagnosis of metabolic syndrome in adolescents. Circulation. 2007;115:2316–22. doi: 10.1161/CIRCULATIONAHA.106.669994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 22.Ventura AK, Loken E, Birch LL. Risk profiles for metabolic syndrome in a nonclinical sample of adolescent girls. Pediatrics. 2006;118:2434–42. doi: 10.1542/peds.2006-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franks PW, Hanson RL, Knowler WC, Moffett C, Enos G, Infante AM, et al. Childhood predictors of young-onset type 2 diabetes. Diabetes. 2007;56:2964–72. doi: 10.2337/db06-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152:201–6. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Weyer C, Tataranni PA, Bogardus C, Pratley RE. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care. 2001;24:89–94. doi: 10.2337/diacare.24.1.89. [DOI] [PubMed] [Google Scholar]
- 26.Haffner SM, Miettinen H, G SP, S MP. Decreased insulin secretion and increased insulin resistance are independently related tot he 7-year risk of NIDDM in Mexican-Americans. Diabetes. 1995;44:1386–91. doi: 10.2337/diab.44.12.1386. [DOI] [PubMed] [Google Scholar]
- 27.Buchanan TA. (How) can we prevent type 2 diabetes? Diabetes. 2007;56:1502–7. doi: 10.2337/db07-0140. [DOI] [PubMed] [Google Scholar]
- 28.Goran MI, Coronges K, Bergman RN, Cruz ML, Gower BA. Influence of family history of type 2 diabetes on insulin sensitivity in prepubertal children. J Clin Endocrinol Metab. 2003;88:192–5. doi: 10.1210/jc.2002-020917. [DOI] [PubMed] [Google Scholar]
- 29.D'Adamo E, Impicciatore M, Capanna R, Loredana Marcovecchio M, Masuccio FG, Chiarelli F, et al. Liver steatosis in obese prepubertal children: a possible role of insulin resistance. Obesity (Silver Spring) 2008;16:677–83. doi: 10.1038/oby.2007.122. [DOI] [PubMed] [Google Scholar]
- 30.Kotronen A, Vehkavaara S, Seppala-Lindroos A, Bergholm R, Yki-Jarvinen H. Effect of liver fat on insulin clearance. Am J Physiol Endocrinol Metab. 2007;293:E1709–15. doi: 10.1152/ajpendo.00444.2007. [DOI] [PubMed] [Google Scholar]
- 31.Park BJ, Kim YJ, Kim DH, Kim W, Jung YJ, Yoon JH, et al. Visceral adipose tissue area is an independent risk factor for hepatic steatosis. J Gastroenterol Hepatol. 2008;23:900–7. doi: 10.1111/j.1440-1746.2007.05212.x. [DOI] [PubMed] [Google Scholar]
- 32.Gastaldelli A, Cusi K, Pettiti M, Hardies J, Miyazaki Y, Berria R, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133:496–506. doi: 10.1053/j.gastro.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 33.Goran MI, Lane C, Toledo-Corral C, Weigensberg MJ. Persistence of pre-diabetes in overweight and obese Hispanic children: association with progressive insulin resistance, poor beta-cell function, and increasing visceral fat. Diabetes. 2008;57:3007–12. doi: 10.2337/db08-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation. 2008;118:277–83. doi: 10.1161/CIRCULATIONAHA.107.739920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Love-Osborne KA, Nadeau KJ, Sheeder J, Fenton LZ, Zeitler P. Presence of the metabolic syndrome in obese adolescents predicts impaired glucose tolerance and nonalcoholic fatty liver disease. J Adolesc Health. 2008;42:543–8. doi: 10.1016/j.jadohealth.2007.11.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Type 2 diabetes in children and adolescents. American Diabetes Association. Diabetes Care. 2000;23:381–9. doi: 10.2337/diacare.23.3.381. [DOI] [PubMed] [Google Scholar]