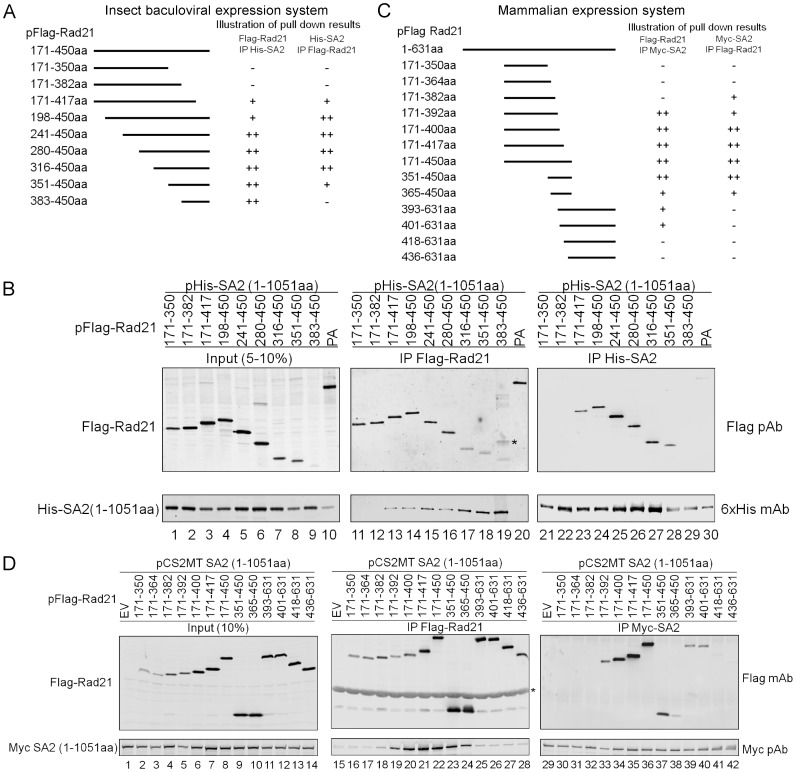
Figure 3. SA2 interacts with a 10 aa region of middle part Rad21.
(A) Schematic illustration of the middle portion Rad21 deletion constructs made in the baculovirus system and interaction results of Rad21-SA2 from (B). ++: strong interaction; +: weak interaction; −: no interaction. (B) Rad21 (171–382 aa) does not interact with SA2. His-SA2 (1–1051 aa) was expressed along with the Flag tagged Rad21 deletion mutants and co-purified with Ni-NTA or Flag beads. Antibody cross-reaction bands are marked by asterisk (*). (C) Schematic illustrations of Rad21 deletion constructs in the context of the full length Rad21 in the mammalian expression vector pFlag CMV2 and interaction results from (D). ++: strong interaction; +: weak interaction; −: no interaction. (D) Rad21 383–392 aa region is critical for interacting with SA2. Myc-SA2 (1–1051 aa) was co-transfected along with the Flag-Rad21 deletion mutants and immunoprecipitated with Flag or Myc beads and probed with either the Myc polyclonal antibody (Myc pAb) or the FLAG mAb. Flag empty vector (EV) was used as a negative control.