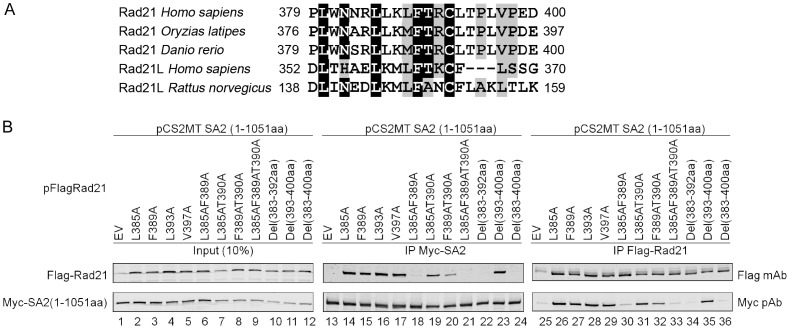
Figure 4. L385 and F389 are critical for Rad21 to interact with SA2.
(A) Sequence alignment of Rad21 from various vertebrate species. The sequence alignment was prepared using BioEdit (http://www.mbio.ncsu.edu/bioedit/). The conserved Rad21 L385, F389 and T390 residues were used for making site-directed mutations. (B) Co-immunoprecipitation analysis indicating the importance of L385, F389, and T390 residues on Rad21 for its interaction with SA2. Flag-Rad21 and Myc-SA2(1–1051 aa) were expressed in 293 T cells. Co-IP was performed 48 h after transfection.