Abstract
Over the past three decades, strictureplasty for Crohn disease with fibrostenotic stricture has been shown to be both efficacious and safe. Although segmental resection remains the standard of care for obstruction secondary to Crohn stricture, strictureplasty should be considered for patients with a history of prior resections who are at increased risk for short bowel syndrome with additional resections. There is ample evidence to support both conventional and nonconventional strictureplasty techniques for both jejunoileal and ileocolonic anastomotic strictures. The role of strictureplasty for both duodenal and colonic disease, as well as the risk of malignant transformation at strictureplasty sites, is yet to be determined.
Keywords: strictureplasty, Crohn disease, stricture, obstruction
Objectives: Upon completion of this article, the reader should be able to (1) describe the indications for strictureplasty, the management of fibrostenotic Crohn disease; (2) describe the surgical techniques for performing intestinal strictureplasty; and (3) have an understanding of the risk of malignant transformation of Crohn-related strictures.
There exist numerous case reports dating back to the early 1800s that describe the entity that we now call Crohn disease (CD), a type of inflammatory bowel disease (IBD) that was formally described by Crohn, Ginzburg, and Oppenheimer in 1932.1,2 CD has been classified as a chronic inflammatory condition thought to have genetic, autoimmune, and environmental components implicated as causative factors; however, more than 80 years after its formal description, the exact causes of CD remain elusive and multifactorial. As a disease process affecting all segments of the gastrointestinal tract, presenting symptoms may range from nonspecific complaints of abdominal pain, diarrhea, weight loss, and gastrointestinal bleeding to more specific complaints resulting from the pathognomonic transmural inflammation of the gastrointestinal wall. These latter symptoms are typically due to disease-specific sequelae, such as fistula, abscess formation, and fibrostenotic strictures, which can be responsible for obstructive symptoms that may necessitate surgical intervention.
Unlike ulcerative colitis that can be cured with surgical resection, the mainstay of treatment for CD is medical management. Mild to moderate disease can be managed with agents such as sulfasalazine, 5-aminosalicylate, antibiotics, and budesonide; more severe symptoms may respond to corticosteroids, anti-tumor necrosis factor (anti-TNF) agents such as infliximab, and immunomodulators such as azathioprine. Despite advances in medical management, more than two-thirds of patients with CD will ultimately require surgical intervention at some point in their lives. Indications for surgical intervention include disease refractory to medical management, complications of medical management, perforation, hemorrhage, perianal sepsis, abscess, obstruction, concern for neoplasia, and severe symptomatology.3
Although the main goal of surgical intervention is to alleviate symptoms, limiting bowel resection is critical to maintaining the absorptive function of the small and large intestines. Removing excessive lengths of intestine with traditional segmental resection can result in short bowel syndrome (SBS), in which patients suffer from malabsorptive symptoms and, in some cases, can lead to serious consequences, including a profound decrease in life expectancy.4 To avoid this, surgical techniques have been developed as an alternative to traditional segmental resection for patients at risk for short bowel syndrome. One such procedure, commonly used as an alternative to bowel resection for stricture, is the technique known as intestinal strictureplasty.
Evolution of Strictureplasty
Strictureplasty techniques were originally developed for the upper gastrointestinal tract, where strictures arose from ulcer disease. Nonresective operative techniques were preferred in this region given the anatomical limitations of the pancreaticobilliary system, which often led to unacceptably high rates of morbidity with more traditional resection methods. The three most common procedures performed for stricturing peptic ulcer disease are the eponymous Heineke-Mikulicz, Finney, and Jaboulay strictureplasties. Rather than undergoing resection or bypass, these procedures allowed for increasing the lumen of the bowel, while avoiding segmental resection.5
The concept of using strictureplasty for multiple small intestinal strictures was first described by Katariya and colleagues in 1977. In an effort to avoid segmental resection in treating multiple tandem tubercular strictures of the intestinal tract, they demonstrated that the use of strictureplasty not only preserved the intestinal absorptive capacity, but was also a safe alternative to segmental resection or bypass.6 This work was followed by Lee and Papaioannou in 1982, who published their use of strictureplasty for the treatment of Crohn strictures in nine patients, eight of whom were successfully treated with either Heineke-Mikulicz or Finney techniques with follow-up ranging from 8 to 42 months.7
Since these initial studies, numerous publications have looked at outcomes of strictureplasty for Crohn stricture, including two recent meta-analyses. In 2007, Yamamoto et al reported on outcome measures for 3,259 strictureplasties (1,112 subjects) performed between 1975 and 2005.8 Specifically, the authors investigated the type of procedure used, most common location for strictureplasty, septic complication rates, postoperative recurrence rates, rates of short bowel syndrome, and the cancer risk involved with this technique. They concluded that the most common technique used for Crohn strictures was the Heineke-Mikulicz technique (81%), often used in the jejunoileal region (94%), where Crohn strictures are most common. Septic complications occurred in 4% of studied cases. The 5-year recurrence rate after jejunoileal or ileocolonic strictureplasty was 28%, with a 3% site-specific recurrence rate. Finally, the authors found the rate of SBS to be extremely low, with only two cases encountered in all the studies included in the systemic review. The cancer risk at the site of strictureplasty was similarly low, with only three case reports encountered in the literature during the period of the study. The authors concluded that strictureplasty is a safe and effective procedure in treating jejunoileal and ileocolonic anastomotic strictures, given the low risk of site-specific recurrence and septic complications.
More recently, in 2012, Campbell et al reviewed 32 studies from 1975 to 2010, encompassing 4,538 strictureplasties among 1,616 subjects.9 Unlike Yamamoto's meta-analysis, which focused on outcome measures, this study compared the efficacy and safety of conventional strictureplasty techniques (Heineke-Mikulicz, Finney) to nonconventional strictureplasty techniques (modified Finney, combined Heineke-Mikulicz and Finney, modified Heineke-Mikulicz, Michelassi, and others). They showed that nonconventional strictureplasty had the same, if not lower rates, of complications compared with the more conventional techniques. Specifically long-term (recurrent stricture, small bowel obstruction, reoperation, carcinoma, and deaths) and short-term (small bowel obstruction, sepsis, postoperative bleed, other infections) complications were analyzed. Early complication rates were 15% for conventional strictureplasty versus 8% for nonconventional strictureplasty, while late complications were 29% for conventional strictureplasty versus 17% for nonconventional strictureplasty. The authors concluded that nonconventional strictureplasty techniques were not inferior to the conventional techniques.
Indications for Strictureplasty
The main indication for strictureplasty is the presence of multiple small bowel strictures within a long segment of bowel. This is especially true for patients who have had prior bowel resection of more than 100 cm in length, and/or those with symptoms of SBS. As with most surgical techniques involving an intestinal anastomosis, preoperative malnutrition is considered a contraindication to performing strictureplasty. Other contraindications to performing strictureplasty include the presence of phlegmon/fistula/perforation at the planned strictureplasty site, stricture next to an already planned resection site, multiple strictures within a small segment, and any suspicion of small bowel malignancy.3 Although the need for a long-segment strictureplasty (Michelassi technique) and strictureplasty in the setting of active disease have traditionally be considered contraindications for strictureplasty in the past, both circumstances have since been proven to be safe and efficacious.10,11,12
Given the limits of preoperative imaging of the small intestine, specific planning for strictureplasty is often made intraoperatively, and is typically based on the location of active disease. For instance, strictures located in the jejunoileal and ileocolonic anastomotic regions have been shown to respond well to strictureplasty techniques, as opposed to duodenal or colonic locations.8 The lack of data supporting strictureplasty in these latter regions appears to be a function of the lower incidence of the disease at these locations, combined with anatomical differences that factor in surgical decision making (vascular supply, tension on segments of bowel, limits of surgical accessibility), and increased malignancy risk. For these reasons, gastrojejunal bypass for CD-related stricturing of the duodenum and segmental resection for colonic stricture have been shown to be more viable alternatives compared with strictureplasty.8,9,13,14
Surgical Techniques
Although several techniques can be used to perform during a strictureplasty, the most commonly used techniques are the Heineke-Mikulicz, Finney, and Michelassi. Typically, the length of the stricture dictates the technique to be used. Most commonly, strictures that are 10 cm or less are best treated using the Heineke-Mikulicz technique, which is the most common strictureplasty performed worldwide.8,9 During this technique, a longitudinal incision is made across the stricture extending into normal bowel on the antimesenteric border, creating an enterotomy. The enterotomy is then closed transversely in either one or two layers using interrupted sutures (Fig. 1).
Fig. 1.
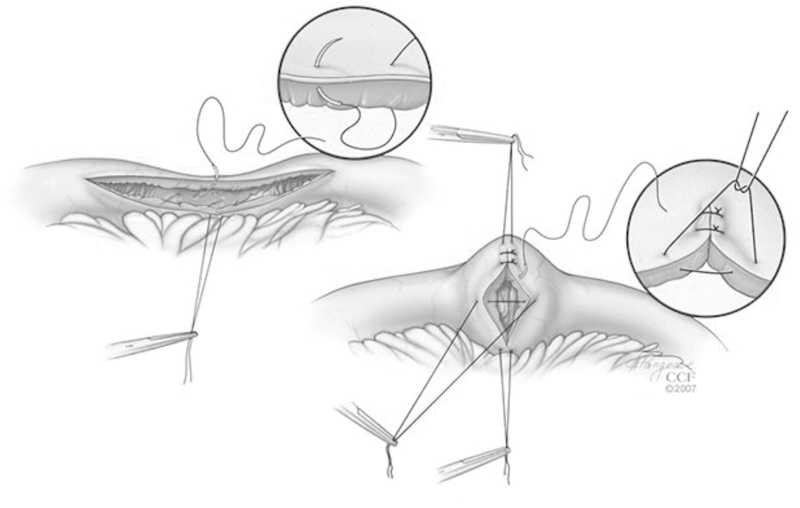
Heineke-Mikulicz strictureplasty. (Reprinted with permission from Cleveland Clinic Center for Medical Art & Photography © 2007-2012. All Rights Reserved.)
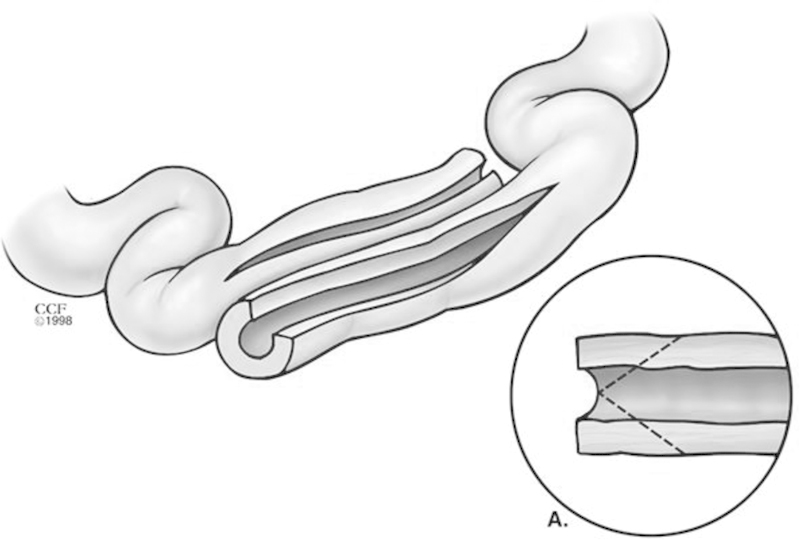
The Finney technique is utilized for medium-sized strictures that are 10 to 20 cm in length. In this technique, a stay suture is first placed in the middle of the stricture, and an antimesenteric enterotomy is performed across the whole length of the stricture that extends into normal bowel. A side-to-side anastomosis is then fashioned between the afferent and efferent limbs of the strictured segment of bowel. A full-thickness running absorbable running suture is first used to reapproximate and take full-thickness bites of the posterior aspect of the enterotomy; then the anterior aspect is closed using interrupted sutures (Fig. 2).
Fig. 2.
Finney strictureplasty. (Reprinted with permission from Cleveland Clinic Center for Medical Art & Photography © 2007-2012. All Rights Reserved.)
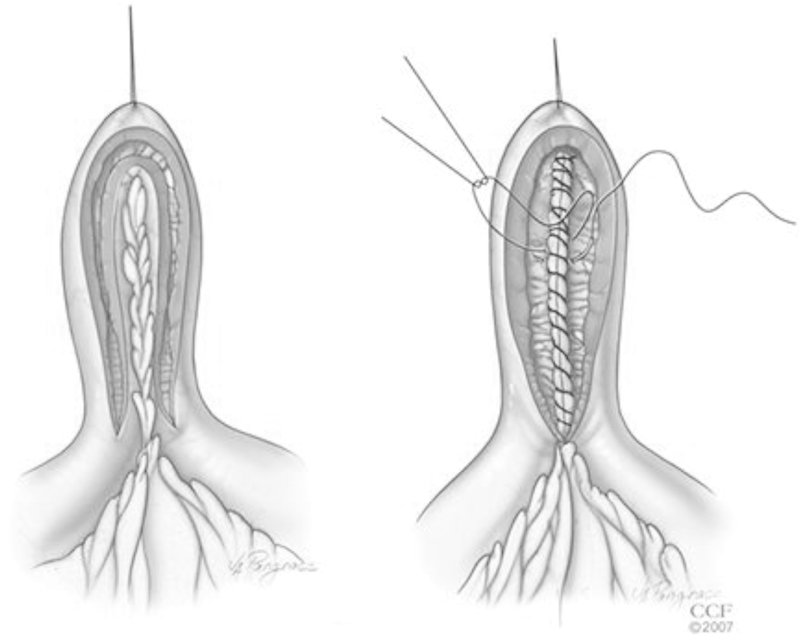
Finally, the Michelassi or side-to-side isoperistaltic strictureplasty is used for long-segment strictures > 20 cm in length. First, the mesentery is divided at the midpoint of the stricture and the two segments of bowel are brought together in a side-to-side fashion approximated with a single layer of interrupted sutures. Following this, an enterotomy is made across the length of the stricture, spatulating the two ends. The inner layer is closed using running full-thickness sutures, while the anterior layer is closed using interrupted sutures (Fig. 3). Several variations and combinations of these techniques are used that make up both conventional and nonconventional strictureplasties, as described by Campbell et al.9 These variations include both handsewn and stapled techniques; the exact technique depends on both the length and location of the stricture(s), which typically will not be completely evident until intraoperative assessment.
Fig. 3.
Michelassi (side-to-side isoperistaltic) strictureplasty. (Reprinted with permission from Cleveland Clinic Center for Medical Art & Photography © 2007-2012. All Rights Reserved.)
Malignant Transformation
The increased risk of malignant transformation in CD has been well documented. A recent meta-analysis of over 60,000 patients with CD found the relative risk of small bowel malignancy to be 28.4 compared with the general population.15 Because of the fear of risk of malignant transformation, some have advocated for segmental resection rather than strictureplasty, performing routine frozen-section analysis before attempting strictureplasty.16 There have been only a handful of documented cases of malignant transformation at or around strictureplasty sites in the published literature. Campbell et al found the rate of small bowel carcinoma to be 0.34% in the conventional strictureplasty group and 0.21% in the nonconventional group.9 The exact site of origin for these cases of small bowel carcinoma is often difficult to ascertain. Although the reported incidence of malignant transformation of a segment of bowel with CD left behind after strictureplasty is very low, the theoretical risk still exists. Therefore, strictureplasty should not be performed in any segment of bowel that appears suspicious for malignant transformation, and in these cases, one may utilize frozen-section analysis before strictureplasty; however, one must keep in mind the risk of sampling error when biopsying a Crohn stricture and the risk of a missed diagnosis of cancer. Further investigation is needed to understand the true risk of malignant transformation at strictureplasty sites, given the high relative risk of small bowel carcinoma in longstanding CD.
Conclusion
Crohn disease is a panintestinal inflammatory disease that has no cure, with the majority of patients requiring one or more surgical intervention during their lifetime. Although segmental resection remains the standard of care for obstruction secondary to Crohn stricture, strictureplasty should be considered for patients with history of prior resections at risk for SBS. Although the majority of the available literature is retrospective reviews comparing a heterogeneous patient population, there is ample evidence to support both the efficacy and safety of conventional and nonconventional strictureplasty techniques for both jejunoileal and ileocolonic anastomotic strictures. The role for strictureplasty for both duodenal and colonic disease, as well as the true incidence of malignant transformation at strictureplasty sites in the setting of longstanding CD, is yet to be determined.
References
- 1.Combe C Saunders W A singular case of stricture and thickening of the ileum Med Trans R Soc Med 180616–18. [Google Scholar]
- 2.Crohn B B, Ginzburg L, Oppenheimer G D. Regional ileitis, pathologic and clinical entity. JAMA. 1932;99:1323–1329. [Google Scholar]
- 3.Beck D E Roberts P L Saclarides T Senagore A Stamos M J Wexner S D The ASCRS Textbook of Colon and Rectal Surgery 2nd ed. New York, NY: Springer; 2011 [Google Scholar]
- 4.Schalamon J, Mayr J M, Höllwarth M E. Mortality and economics in short bowel syndrome. Best Pract Res Clin Gastroenterol. 2003;17(6):931–942. doi: 10.1016/s1521-6918(03)00079-9. [DOI] [PubMed] [Google Scholar]
- 5.Cameron J Cameron A Current Surgical Therapy 10th ed. Philadelphia, PA: Elsevier; 2011 [Google Scholar]
- 6.Katariya R N, Sood S, Rao P G, Rao P L. Stricture-plasty for tubercular strictures of the gastro-intestinal tract. Br J Surg. 1977;64(7):496–498. doi: 10.1002/bjs.1800640713. [DOI] [PubMed] [Google Scholar]
- 7.Lee E C, Papaioannou N. Minimal surgery for chronic obstruction in patients with extensive or universal Crohn's disease. Ann R Coll Surg Engl. 1982;64(4):229–233. [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto T, Fazio V W, Tekkis P P. Safety and efficacy of strictureplasty for Crohn's disease: a systematic review and meta-analysis. Dis Colon Rectum. 2007;50(11):1968–1986. doi: 10.1007/s10350-007-0279-5. [DOI] [PubMed] [Google Scholar]
- 9.Campbell L, Ambe R, Weaver J, Marcus S M, Cagir B. Comparison of conventional and nonconventional strictureplasties in Crohn's disease: a systematic review and meta-analysis. Dis Colon Rectum. 2012;55(6):714–726. doi: 10.1097/DCR.0b013e31824f875a. [DOI] [PubMed] [Google Scholar]
- 10.Hurst R D, Michelassi F. Strictureplasty for Crohn's disease: techniques and long-term results. World J Surg. 1998;22(4):359–363. doi: 10.1007/s002689900397. [DOI] [PubMed] [Google Scholar]
- 11.Michelassi F, Hurst R D, Melis M. et al. Side-to-side isoperistaltic strictureplasty in extensive Crohn's disease: a prospective longitudinal study. Ann Surg. 2000;232(3):401–408. doi: 10.1097/00000658-200009000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roy P, Kumar D. Strictureplasty. Br J Surg. 2004;91(11):1428–1437. doi: 10.1002/bjs.4804. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto T, Bain I M, Connolly A B, Allan R N, Keighley M R. Outcome of strictureplasty for duodenal Crohn's disease. Br J Surg. 1999;86(2):259–262. doi: 10.1046/j.1365-2168.1999.01022.x. [DOI] [PubMed] [Google Scholar]
- 14.Sampietro G M Cristaldi M Maconi G et al. A prospective, longitudinal study of nonconventional strictureplasty in Crohn's disease J Am Coll Surg 200419918–20., discussion 20-22 [DOI] [PubMed] [Google Scholar]
- 15.von Roon A C, Reese G, Teare J, Constantinides V, Darzi A W, Tekkis P P. The risk of cancer in patients with Crohn's disease. Dis Colon Rectum. 2007;50(6):839–855. doi: 10.1007/s10350-006-0848-z. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro M B, Greenstein A J, Heimann T M, Yamazaki Y, Aufses A H Jr. Adenocarcinoma of the small intestine in Crohn's disease. Surg Gynecol Obstet. 1991;173(5):343–349. [PubMed] [Google Scholar]


