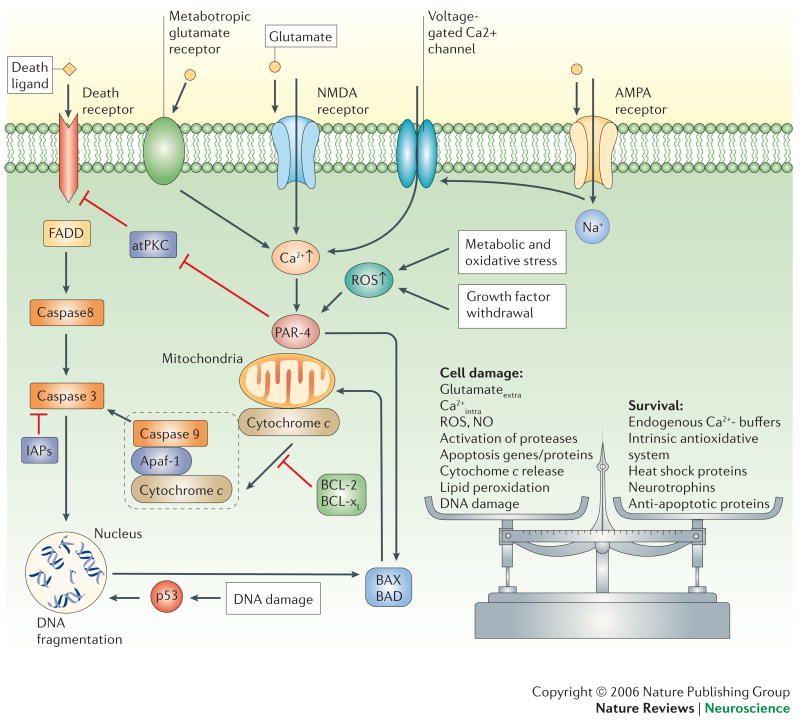
Figure 4. Once triggered, the death of neurons is programmed.
Death signals activate intracellular cascades involving increased levels of ROS and Ca2+, production of Par-4 (prostate apoptosis response-4) and p53, and translocation of pro-apoptotic Bcl-2 family members (Bax and Bad) to the mitochondrial membrane. These events are followed by increased mitochondrial dysregulation and release of cytochrome c into the cytosol. Cytochrome c forms a complex with apoptotic protease-activating factor 1 (Apaf-1) and caspase-9. Activated caspase-9 cleaves and activates caspase-3 which, in turn cleaves protein substrates that effect changes in the plasma membrane, cytoskeleton and nucleus. Certain caspases (caspase-8, for example) can also be directly activated through death ligands and can act independently of mitochondrial changes. The process of apoptosis can be inhibited at different stages through anti-apoptotic mechanisms such as IAPs (inhibitor of apoptosis proteins) or Bcl2 and Bcl-xl. In general, cell fate is decided by a balance between survival factors and potentially harmful or destructive factors. atPKC, atypical protein kinase C; FADD, Fas-associated death domain protein.