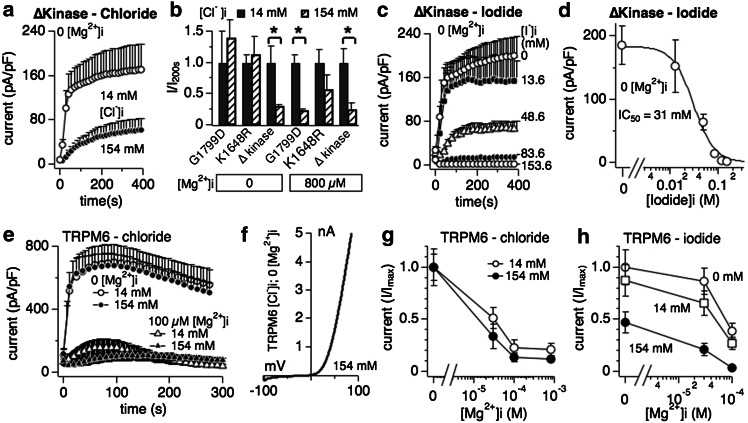
Fig. 4.
Interaction of halides with TRPM7 delta kinase channels and TRPM6. a Whole-cell patch clamp experiments in tetracyclin-inducible HEK293 cells overexpressing TRPM7 truncated kinase mutant (∆kinase) [7]. Cells were perfused with intracellular solution containing either 14 mM or 154 mM Cl− and in the absence of intracellular Mg2+ (n = 6–8). See “Methods” for solution details. All currents were assessed at +80 mV during a voltage-ramp stimulus ranging from −100 to +100 mV over 50 ms and given at 0.5-Hz intervals. Currents were averaged and normalized to the cell capacitance (in pF) recorded at whole-cell break-in. Error bars indicated SEM. b The bar graph analysis shows normalized current amplitudes measured at +80 mV and at 200 s for three different TRPM7 kinase domain mutants (G1799D, K1648R and ∆kinase) and normalized as I/Imax(200s). Internal solutions were kept at low (14 mM, black bars) or high (154 mM, diagonally striped bars) chloride and were supplemented with 800 μM [Mg2+]i or not as indicated in the graph. c Inhibition of TRPM7 ∆kinase currents by increasing intracellular I− concentrations in the absence of intracellular Mg2+ (n = 6–8). d Dose–response curve analysis of TRPM7 currents shown in panel c. Data points correspond to average current amplitudes measured at +80 mV at 200 s of whole-cell recording, plotted as a function of l− concentration from panel c (n = 6–8). The best fit was obtained by a Hill coefficient close to −3 and was therefore fixed at that value. The calculated IC50 value for l−-induced inhibition of TRPM7 ∆kinase currents is IC50 = 31 ± 3 mM. e Assessment of chloride inhibition on human TRPM6 (hTRPM6) transiently expressed between 26 and 30 h in wild-type HEK293 cells (see “Methods”). Currents were elicited by standard voltage ramps, assessed at +80 mV, averaged, normalized for cell size (in pF) and plotted versus time of the experiment. f Representative I/V curve for TRPM6 in the presence of intracellular 154 mM Cl− and without internal Mg2+, taken from experiments in panel e. All I/V relationships were taken at 75 s and were derived from a high-resolution current record in response to a voltage ramp of 50 ms duration that ranged from −100 to +100 mV. g Cells transiently expressing hTRPM6 were perfused with internal solutions containing either 14 or 154 mM Cl− and increasing intracellular Mg2+ concentrations. Data shown were measured at +80 mV, assessed at 75 s whole-cell time and normalized to I/Imax (n = 6–8). h Dose-dependent inhibition of hTRPM6 currents plotted as a function of Mg2+ concentrations assessed at different fixed I− concentrations. Data points correspond to average current amplitudes measured at +80 mV and 75 s of whole cell recording (n = 4–8). Data were normalized to I/Imax