Abstract
Mechanical load-induced intracellular signaling events are important for subsequent skeletal muscle hypertrophy. We previously showed that load-induced activation of the cation channel TRPV1 caused an increase in intracellular calcium concentrations ([Ca2+]i) and that this activated mammalian target of rapamycin (mTOR) and promoted muscle hypertrophy. However, the link between mechanical load-induced intracellular signaling events, and the TRPV1-mediated increases in [Ca2+]i are not fully understood. Here we show that administration of the TRPV1 agonist, capsaicin, induces phosphorylation of mTOR, p70S6K, S6, Erk1/2 and p38 MAPK, but not Akt, AMPK or GSK3β. Furthermore, the TRPV1-induced phosphorylation patterns resembled those induced by mechanical load. Our results continue to highlight the importance of TRPV1-mediated calcium signaling in load-induced intracellular signaling pathways.
Keywords: skeletal muscle, muscle hypertrophy, calcium signaling, TRPV1, capsaicin, mTOR
Introduction
Skeletal muscle mass is regulated by a balance of protein synthesis and degradation. Increased muscle activity by exercise or weight training induces activation of mammalian target of rapamycin (mTOR), which promotes protein synthesis and subsequent muscle hypertrophy.1 Insulin-like growth factor-1 (IGF-1) activates mTOR through the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, which is important for muscle growth and/or muscle hypertrophy.2 However, recent studies have revealed that mechanical load-induced activation of mTOR was not mediated by PI3K/Akt, especially at early stages of muscle hypertrophy,1,3-5 suggesting the presence of another route that converts mechanical load into the activation of mTOR. We previously showed that mechanical load-induced activation of mTOR and subsequent muscle hypertrophy required a TRPV1-mediated increase in intracellular calcium concentrations ([Ca2+]I),6 implicating calcium signaling as a crucial step for mechanical load-induced activation of mTOR. However, it is still unclear that to what extent the TRPV1-mediated increase in [Ca2+]i is involved in intracellular signaling after mechanical load. Here, we show additional data that further highlight the significance of the calcium-induced intracellular signaling in response to mechanical load in skeletal muscle.
Results and Discussion
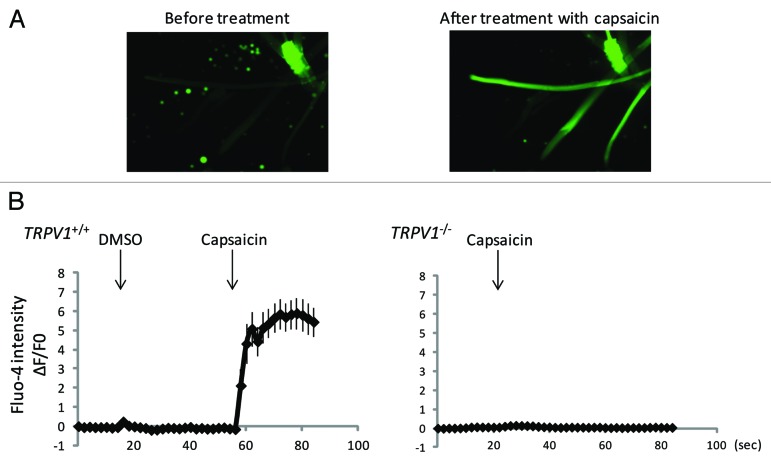
To investigate the effect of TRPV1-induced calcium signaling on intracellular signaling events, we treated muscle cells with the TRPV1 agonist, capsaicin. We isolated single muscle fibers from the extensor digitorum longus muscle of wild-type or TRPV1-null mice and analyzed the effect of capsaicin on [Ca2+]i using Fluo-4. [Ca2+]i were increased by capsaicin in wild-type but not in TRPV1-null muscle fibers, indicating that the capsaicin-induced increases in [Ca2+]i were mediated by TRPV1 (Fig. 1A and B).
Figure 1. Capsaicin induces an increase of intracellular calcium levels in a TRPV1-dependent manner. (A) Ca2+-imaging using Fluo-4 fluorescence of isolated wild-type single muscle fibers from extensor digitorum longus before or after treatment with capsaicin. (B) Quantitative analysis of Fluo-4 signal intensity. Approximately 10 fibers were analyzed.
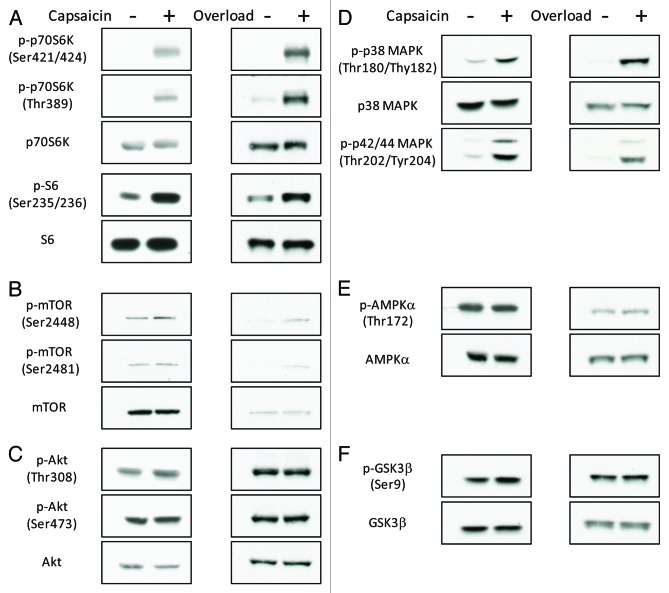
We previously showed that mTOR was activated by the TRPV1-induced increases in [Ca2+]i6. Because [Ca2+]i regulates divergent intracellular signaling pathways,7-9 we further analyzed the capsaicin-induced intracellular signaling events. Similar to previous observations,6 intramuscular injection of capsaicin induced phosphorylation of p70S6K at Thr389, an mTOR-regulated phosphorylation site that is necessary for p70S6K activation10 in addition to phosphorylation of the downstream target S6 (Fig. 2A). Capsaicin also induced phosphorylation of p70S6K at Thr421/Ser424 and mTOR at Ser2448 and Ser2481. In addition to p70S6K, capsaicin induced phosphorylation of Erk1/2 and p38 MAPK, but not Akt, AMPKα, or GSK3β (Fig. 2B–F). Interestingly, similar changes in phosphorylation were observed within 3 min of mechanical overload, an experimental model of hypertrophy, which relies on compensatory adaptation of the plantaris muscle following ablation of the tendons of the functionally synergistic muscles (Fig. 2). This suggests that load-induced intracellular signaling is regulated by TRPV1-mediated increase in [Ca2+]i.
Figure 2. Mechanical overload and administration of capsaicin induce phosphorylation of p70S6K, S6, mTOR, Erk1/2 and p38 MAPK, but not Akt, AMPKα or GSK3β. Western blot analysis showing the effects of mechanical overload or administration of capsaicin on phosphorylation of p70S6K and S6 (A), mTOR (B), Akt (C), Erk1/2 and p38 MAPK (D), AMPKα (E) and GSK3β (F) (n = 3–4).
Unlike the IGF-1-induced activation of the PI3K/Akt/mTOR pathway,2 the load- or the TRPV1-induced activation of mTOR occurred independently of the phosphorylation of Akt (Fig. 2C). IGF-1 is responsible for the induction of muscle hypertrophy.11 However, because transgenic mice that overexpressed a dominant-negative IGF-1 receptor showed normal overload-induced hypertrophy,4 and relatively early activation of mTOR induced by overload occurred independently of PI3K/Akt signaling,3 at least one other signaling pathway is likely to be present. Because capsaicin mimicked overload-induced activation of intracellular signaling events (Fig. 2), and we previously showed that treatment with capsaicin was sufficient to induce hypertrophy,6 our study suggests the activation of TRPV1-mediated increase in [Ca2+]i to be an important step in the induction of hypertrophy. Activation of the MAPK pathway upregulates several transcription factors.12 Furthermore, previous studies indicate that mTOR is regulated by Erk1/2-induced regulation of TSC1/2.3,9 A loss-of-function approach for Erk1/2 will be required to investigate any molecular link between a potential load-induced activation of the Erk1/2 pathway and the mTOR pathway. The elucidation of the molecular mechanisms by which mechanical load activates mTOR, Erk1/2 and p38 MAPK will lead to a better understanding of the mechanisms underlying load-induced muscle hypertrophy and might contribute to the development of therapeutics to counteract muscle atrophy. Furthermore, elucidation of the mechanisms involved in co-activation of these molecules by mechanical load and TRPV1-induced regulation of [Ca2+]i will lead to a better understanding of load-induced muscle hypertrophy.
Materials and Methods
Animals
Twelve- to forty-week-old male TRPV1-null mice and C57BL/6 mice were purchased from Charles River Laboratories and Nihon CREA, respectively. These mice were housed at the institutional animal facility. A 150 μl of a 10 μM solution of capsaicin (Sigma-Aldrich) was injected intramuscularly 30 min before isolation of muscles.
Isolation of single fibers and intracellular calcium level measurement
Extensor digitorum longus muscles from wild-type and TRPV1-null mice were isolated and dissociated by digestion using type 1 collagenase in a buffer containing 140 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 10 mM glucose (pH 7.0). [Ca2+]i were monitored with Fluo-4 as previously described.6 Briefly, single fibers were incubated with 4 μM Fluo-4 a.m. (Dojindo) for 30 min at room temperature to allow homogenous intracellular distribution of the dye. After raising the temperature to 37 °C, the fibers were placed onto the stage of an inverted microscope (Olympus), and fluorescence intensity changes by vehicle (DMSO) or capsaicin (10 μM) were recorded every 2 sec. Data were calculated as normalized fluorescence ΔF/F0: ΔF/F0 = (Fmax - F0)/F0, where Fmax is the maximum fluorescence and F0 is the fluorescence before exposure to a reagent.
Western blot analysis
Total muscle protein was extracted by sample buffer containing 0.1% Triton X-100, 50 mM HEPES (pH 7.4), 4 mM EGTA, 10 mM EDTA, 15 mM Na4P2O7, 100 mM glycerophosphate, 25 mM NaF, 5 mM Na2VO4 and a complete protease inhibitor cocktail (Roche). The protein concentration was determined using Coomassie brilliant blue G-250 (Bio-Rad). Just before SDS-PAGE, an aliquot of the extracted protein solution was mixed with an equal volume of sample loading buffer containing 30% glycerol, 5% 2-mercaptoethanol, 2.3% SDS, 62.5 mM TRIS-HCl (pH 6.8) and 0.05% bromophenol blue, and the mixture was heated at 60 °C for 10 min. Thirty μg of protein was separated on an SDS-polyacrylamide gel and electrically transferred to a polyvinylidene difluoride membrane (Millipore). The blot was incubated with primary antibodies. The signals were detected using the ECLTM Western Blotting Detection system (GE Healthcare). Antibodies against Akt (9272), phospho-Akt (Ser473) (9271), phospho-Akt (Thr308) (9275), p70S6K (9202), phospho-p70S6K (Thr389) (9205), phospho-p70S6K (Thr421/Ser424) (9204), S6 (2217), phospho-S6 (Ser235/236) (4858), mTOR (2972), phospho-mTOR (Ser2448) (2971), phospho-mTOR (Ser2481) (2974), AMPKα (2603), phospho-AMPKα (Thr172) (2535), phospho-ERK1/2 (Thr202/Tyr204) (9101), p38 MAPK (9212), phospho-p38 MAPK (Thr180/Tyr182) (9211), phospho-GSK-3β (Ser9) (9336) were purchased from Cell Signaling Technology. Antibody against GSK-3β (sc-9166) was purchased from Santa Cruz.
Synergist ablation surgery
Mice were anesthetized with diethyl ether. Functional overload of the plantaris muscle was induced by bilateral surgical ablation of the tendons of the gastrocnemius and soleus muscles. Briefly, a midline incision was made in the skin on the hind limbs. The distal tendons of both the gastrocnemius and soleus were transected. The incision was closed with a 7–0 silk suture (Matsuda Ika Kogyo). Plantaris muscles were isolated immediately 3 min after mice recovered from the anesthetic and started walking. For the sham-operated group, similar incisions were made in the skin, but the tendons were not transected.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (B) and a Grant-in-Aid for JSPS Fellows.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/24583
References
- 1.Philp A, Hamilton DL, Baar K. Signals mediating skeletal muscle remodeling by resistance exercise: PI3-kinase independent activation of mTORC1. J Appl Physiol. 2011;110:561–8. doi: 10.1152/japplphysiol.00941.2010. [DOI] [PubMed] [Google Scholar]
- 2.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, et al. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–13. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 3.Miyazaki M, McCarthy JJ, Fedele MJ, Esser KA. Early activation of mTORC1 signalling in response to mechanical overload is independent of phosphoinositide 3-kinase/Akt signalling. J Physiol. 2011;589:1831–46. doi: 10.1113/jphysiol.2011.205658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spangenburg EE, Le Roith D, Ward CW, Bodine SC. A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy. J Physiol. 2008;586:283–91. doi: 10.1113/jphysiol.2007.141507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hornberger TA, Stuppard R, Conley KE, Fedele MJ, Fiorotto ML, Chin ER, et al. Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem J. 2004;380:795–804. doi: 10.1042/BJ20040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito N, Ruegg UT, Kudo A, Miyagoe-Suzuki Y, Takeda S. Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat Med. 2013;19:101–6. doi: 10.1038/nm.3019. [DOI] [PubMed] [Google Scholar]
- 7.Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, et al. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 2008;7:456–65. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamás P, Hawley SA, Clarke RG, Mustard KJ, Green K, Hardie DG, et al. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006;203:1665–70. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wauson EM, Zaganjor E, Lee AY, Guerra ML, Ghosh AB, Bookout AL, et al. The G protein-coupled taste receptor T1R1/T1R3 regulates mTORC1 and autophagy. Mol Cell. 2012;47:851–62. doi: 10.1016/j.molcel.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–18. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 11.Adams GR, Haddad F. The relationships among IGF-1, DNA content, and protein accumulation during skeletal muscle hypertrophy. J Appl Physiol. 1996;81:2509–16. doi: 10.1152/jappl.1996.81.6.2509. [DOI] [PubMed] [Google Scholar]
- 12.Hazzalin CA, Mahadevan LC. MAPK-regulated transcription: a continuously variable gene switch? Nat Rev Mol Cell Biol. 2002;3:30–40. doi: 10.1038/nrm715. [DOI] [PubMed] [Google Scholar]