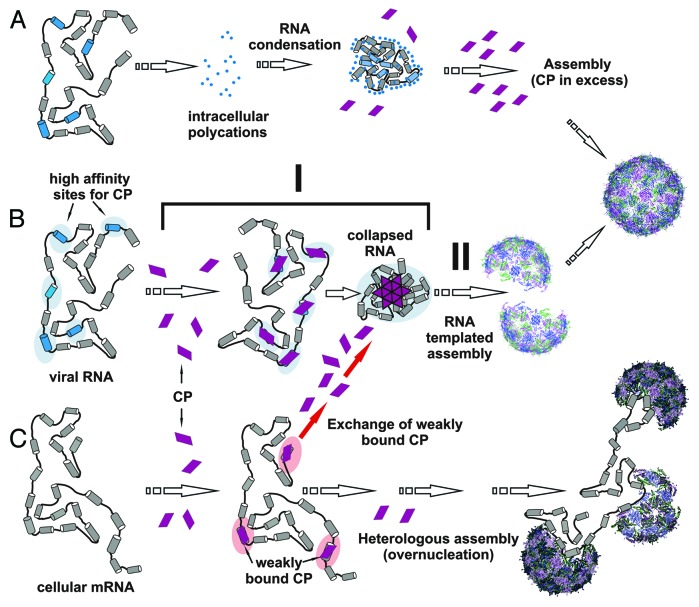
Figure 4. The two-stage assembly mechanism for viral RNA. Our results suggest that there are three forms of RNA condensation/compaction in the context of virus assembly. (A) Non-specific condensation of viral RNA by multivalent ions (Mg2+, spermidine) inhibits assembly by coat proteins (middle). Only at very high CP concentrations (> 5 μM) is this block overcome (right), showing that simple electrostatic condensation is not on pathway to capsid formation. (B) A two-stage mechanism of assembly in which CPs first bind to cognate RNAs displaying multiple packaging sites (blue segments), distributed throughout the viral genome to facilitate protein-protein interactions, thus mediating a rapid RNA collapse (stage I). The collapse is followed by cooperative recruitment of additional CP subunits (stage II), even at low concentration (< 1 μM), to complete capsid assembly. (C) Assembly with non-cognate (cellular) RNAs leads to weak interactions without observable initial RNA collapse. Dissociation of coat proteins from these complexes allows them to be captured by the cognate assembly pathway (red arrows, middle). A low yield alternative pathway occurs when non-specifically bound coat proteins nucleate assembly on cellular RNA. Since the coat protein binding sites are not correctly positioned, these RNAs do not collapse and there can be multiple nucleation events leading to misassembled and multishell structures. Such pathways explain the assembly of non-cognate RNAs in vitro at relatively high coat protein concentrations.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.