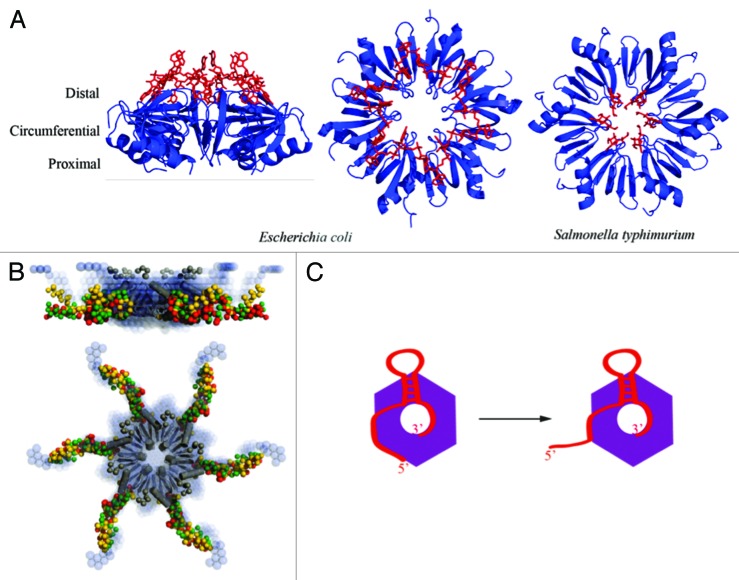
Figure 4. The structure and proposed RNA-binding modes of the RNA chaperone Hfq. (A) Crystal structures revealing the ring-like architecture of the hexameric Hfq protein and RNA binding to the distal (left and central panels, E. coli)98 and proximal faces (right panel, Salmonella typhimurium)99 of the ring. (B) C-terminal tails emanate from the core, based on small angle X-ray solution scattering. These tails have some weak conservation in families and are likely to be flexible.100,101 They may help to capture RNA and accommodate species with complex folds38 or serve for interaction with other proteins.102 (C) A proposed binding model for sRNA (red) in which the 3′ poly(U) tail is engaged on the proximal side of the Hfq hexamer (purple), and the body of the sRNA is engaged on the circumferential rim of the ring.99,103 The mRNA recognition region, here located on the 5′ end of the sRNA, is proposed to be able to peel off to mediate cognate base pairing with the target mRNA.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.