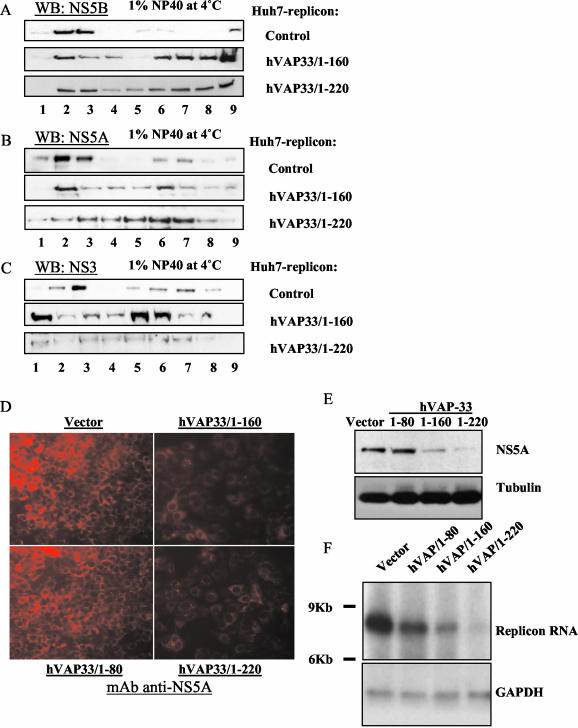
FIG. 5.
Effects of tuncation mutants of hVAP-33 on association of HCV NS proteins with detergent-resistant membrane fractions. (A to C) Membrane flotation analysis. Huh7-replicon cells transfected with hVAP-33/1-160 or hVAP-33/1-220 were harvested at 3 days posttransfection. Cell lysates were treated with 1% NP-40 for 1 h on ice and then fractionated by discontinuous sucrose gradient centrifugation. (D) Immunofluorescence staining of NS5A in Huh7-replicon cells at 4 days posttransfection. Cells seeded on chamber slides were fixed in 4% formaldehyde-PBS, permeabilized by 0.1% Triton X-100-PBS, and immunostained with monoclonal antibodies to NS5A, followed by the addition of secondary antibodies conjugated with TRITC. Images were taken under fluorescence microscopy with the Zeiss program for the same length of exposure time for all panels. (E) Western blot analysis of protein expression in transfected Huh7-replicon cells at 4 days posttransfection. Thirty micrograms of postnuclear cell lysate from each transfection was separated by SDS-PAGE, transferred to nitrocellulose membrane, and probed with monoclonal antibody to NS5A. The same membrane was reprobed with anti-tubulin antibody as loading control. (F) Northern blot analysis of total RNA extracted from Huh7-replicon cells transfected with the various plasmids expressing truncated hVAP-33 or vector at 3 days posttransfection. Ten micrograms of total RNA from each plate was used. A specific RNA probe complementary to the HCV 5′-UTR RNA was used in hybridization. GADPH served as a loading control.