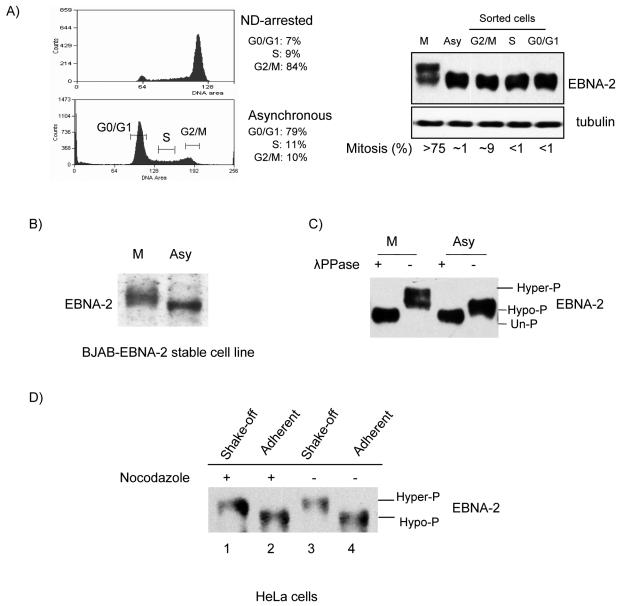
FIG. 1.
EBNA-2 is hyperphosphorylated specifically in mitosis. (A) Left top panel, X50-7 cells arrested by nocodazole (ND); left bottom panel, G0/G1, S and G2/M phases sorted from asynchronously growing cells by FACS (brackets indicate collected fractions). Percentages in each phase are indicated. Right panel, EBNA-2 protein analyzed by immunoblotting with PE2 antibody; tubulin was the loading control. The mitotic index was determined by DAPI staining. (B) Immunoblots of EBNA-2 stably expressed in EBV-negative BJAB cells in nocodazole-arrested M phase (M) or in asynchronous cells (Asy) in whole-cell lysates. (C) Hyperphosphorylation of EBNA-2 in nocodazole-arrested M-phase cells. Immunoprecipitated EBNA-2 from M-phase and asynchronous cells was subjected to immunoblotting directly (−) or after treatment with 2,000 U of λ phosphatase (λPPase) (+). (D) EBNA-2 is hyperphosphorylated in both nocodazole-arrested and untreated mitotic cells. HeLa cells were transfected with EBNA-2 and treated with nocodazole (250 ng/ml) for 18 h, at 20 h after transfection, or were left untreated. M-phase cells were separated from other phases by vigorous shaking. Immunoblots for EBNA-2 of extracts from mitotic cells collected by shake-off from nocodazole-arrested (lane 1) and untreated (lane 3) cell monolayers and from cells remaining adherent (lanes 2 and 4) were analyzed.