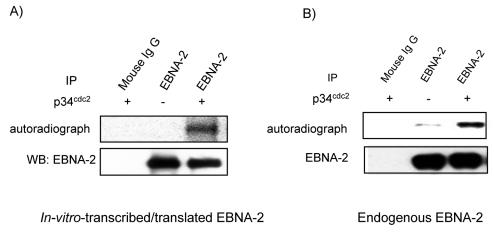
FIG. 3.
Involvement of p34cdc2 kinase in EBNA-2 phosphorylation. In vitro-transcribed-translated EBNA-2 from rabbit reticulocyte lysates (A) or endogenous EBNA-2 from total lysates of asynchronously growing cells (B) were immunoprecipitated from cells and used as substrates for kinase assays in the presence (+) or absence (−) of purified p34cdc2/cyclin B1 kinase. Immunoprecipitation with normal mouse IgG served as the negative control. After resolving by electrophoresis and transfer onto membrane, phosphorylation of EBNA-2 was detected by autoradiography (top panel). Equal levels of EBNA-2 were confirmed by immunoblotting (bottom panel).