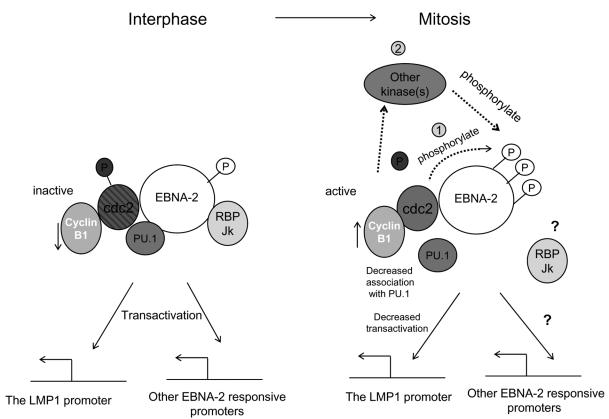
FIG. 7.
Mitotic hyperphosphorylation of EBNA-2 and its possible functional consequences. In the interphase, p34cdc2 kinase is inactive due to the low level of cyclin B1, its regulatory subunit, and phosphorylation of p34cdc2. EBNA-2 is hypophosphorylated in the interphase and transcriptionally active. Upon entering the M phase of the cell cycle, p34cdc2 kinase becomes activated by accumulation of cyclin B1 and dephosphorylation of p34cdc2. EBNA-2 is hyperphosphorylated during mitosis by p34cdc2 kinase directly (1) and probably by other kinases as well (2). Association of EBNA-2 with PU.1 decreases, which may be one of the mechanisms whereby transactivation of the LMP-1 promoter by EBNA-2 is impaired. In general, the suppression of EBNA-2 transcriptional activity by hyperphosphorylation may repress all the other EBNA-2-responsive genes during the M phase of the cell cycle.