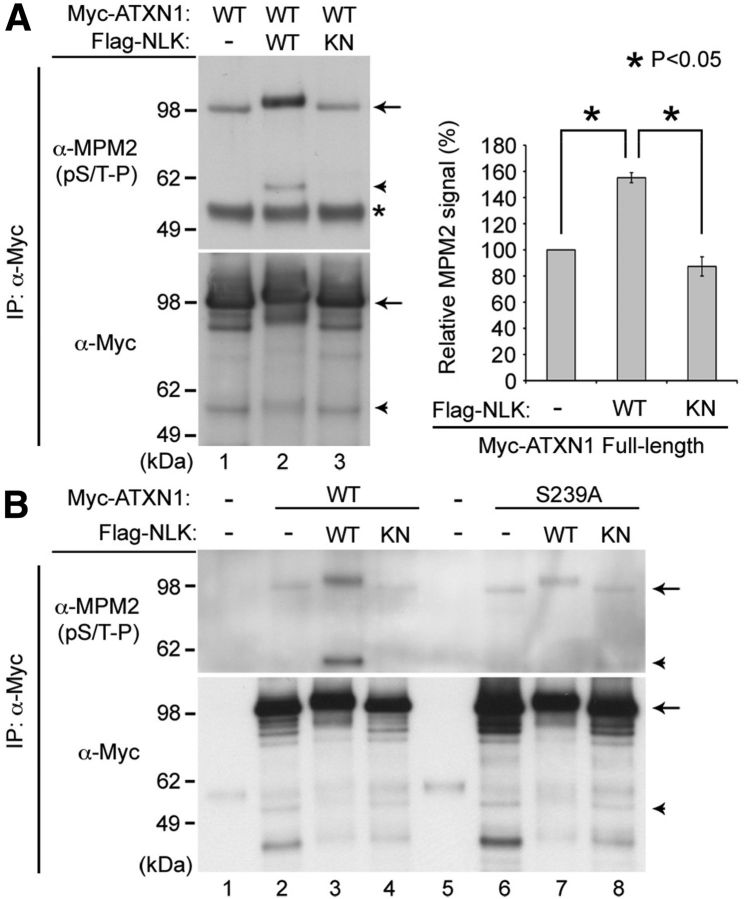
Figure 2.
NLK phosphorylates ATXN1. A, NLK increases phosphorylation of ATXN1 at S/T-P sites. B, The S239 residue in ATXN1 is an NLK phosphorylation site. HEK293T cells were transfected with N-terminal-tagged myc-ATXN1(30Q) and WT, kinase-inactive mutant (KN) Nlk, or a control plasmid (−). The phosphorylation of full-length (arrows) or an N-terminal fragment (∼55 kDa in size, arrowheads) of WT ATXN1 is strongly increased by WT NLK, but not by the KN mutant. *Immunoglobin heavy chain used for immunoprecipitation (IP). S239A is a serine to alanine substitution at residue 239. Normalized levels of MPM2 signal from the full-length ATXN1 are shown (A, right). Mean relative levels (control plasmid (−) = 100%) and SE are shown (n = 2). *p < 0.05 (t test).