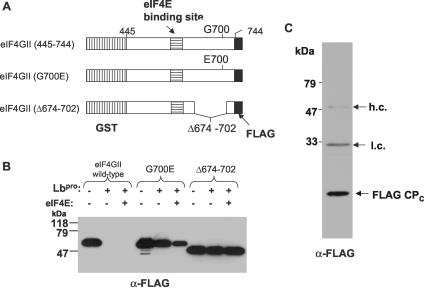
FIG. 4.
In vitro cleavage of a fragment of eIF4GII by FMDV Lbpro permits identification of the cleavage site. (A) Structure of the GST-eIF4GII-FLAG (abbreviated GST-4GII-FLAG) fusion proteins as described previously (14). (B) Recombinant GST-4GII-FLAG protein (2.5 μg) or mutant forms with a G700E substitution or deletion of residues 674 to 702 were incubated in the presence (+) or absence (−) of recombinant FMDV Lbpro (1 μg) alone or with recombinant eIF4E (1 μg) as indicated. Samples were analyzed by SDS-PAGE (15% polyacrylamide) and immunoblotting with anti-FLAG M2 antibodies. Detection was achieved using chemiluminescence reagents. (C) The C-terminal cleavage product of the wt GST-4GII-FLAG protein, generated by the FMDV Lbpro, was isolated using an anti-FLAG M2 affinity resin. An aliquot of the material was analyzed by SDS-PAGE (15.5% polyacrylamide) and immunoblotting with anti-FLAG M2 antibodies as for panel B. The presence of antibody heavy chains (h.c.) and light chains (l.c.) as well as the FLAG-tagged C-terminal cleavage product (CPC) is indicated.