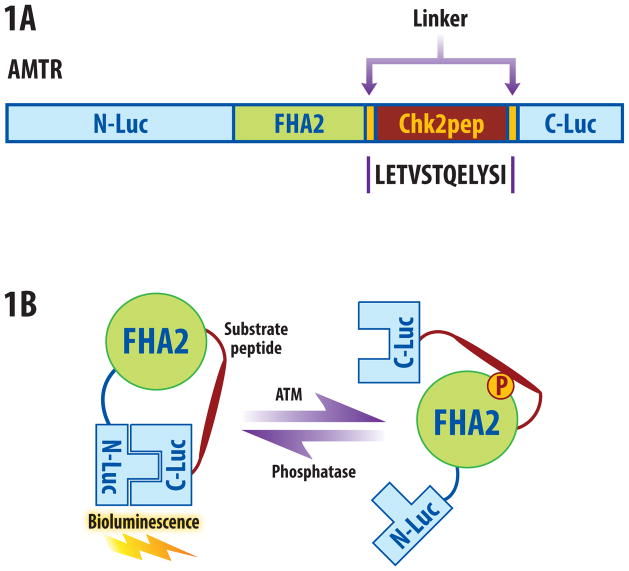
Figure 1.
ATM reporter (ATMR). (a) The domain structure of the ATMR. The N-Luc and C-Luc fragments of firefly luciferase flank an FHA2 phosphopeptide binding domain and 12 amino acid Chk2 substrate peptide. (b) The proposed mechanism of action for the ATM reporter involves ATM-dependent phosphorylation of the Chk2 peptide (thick line), which results in interaction of the FHA2 domain (right). In this form, the reporter has minimal bioluminescence activity. In the absence of ATM kinase activity, the N-Luc and C-Luc domains re-associate, restoring bioluminescence activity (left).