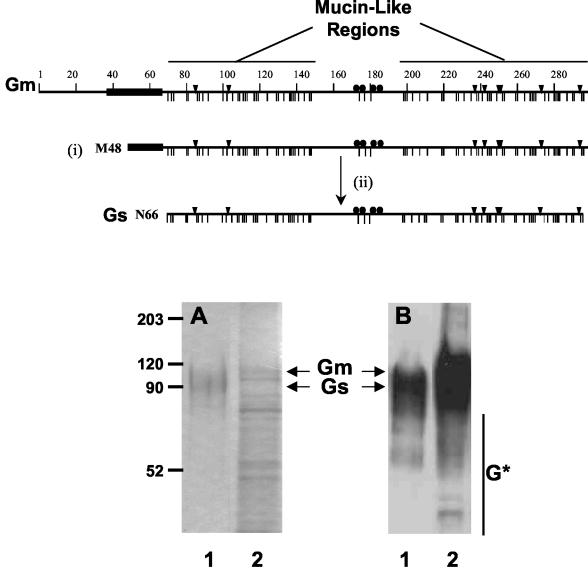
FIG. 1.
SDS-PAGE and Western blotting of Gs and Gm proteins. A scheme of the Gm protein is presented in the upper part of the figure, denoting the hydrophobic region ( ), the potential N- (▾) and O-glycosylation sites (|), and the cysteine residues (•). Also indicated are the two mucin-like regions. Formation of the Gs form occurs by translation initiation (i) at Met48 and subsequent cleavage (ii) after residue 65. HRSV Gs (lanes 1) and Gm (lanes 2) proteins were purified by immunoaffinity chromatography, as described in Materials and Methods, and analyzed by Coomassie staining of an SDS-PAGE gel (A) and by Western blotting with monoclonal antibody 63G (B). Molecular weight markers are shown on the left. G* indicates low-molecular-weight bands reacting with antibody 63G.
), the potential N- (▾) and O-glycosylation sites (|), and the cysteine residues (•). Also indicated are the two mucin-like regions. Formation of the Gs form occurs by translation initiation (i) at Met48 and subsequent cleavage (ii) after residue 65. HRSV Gs (lanes 1) and Gm (lanes 2) proteins were purified by immunoaffinity chromatography, as described in Materials and Methods, and analyzed by Coomassie staining of an SDS-PAGE gel (A) and by Western blotting with monoclonal antibody 63G (B). Molecular weight markers are shown on the left. G* indicates low-molecular-weight bands reacting with antibody 63G.