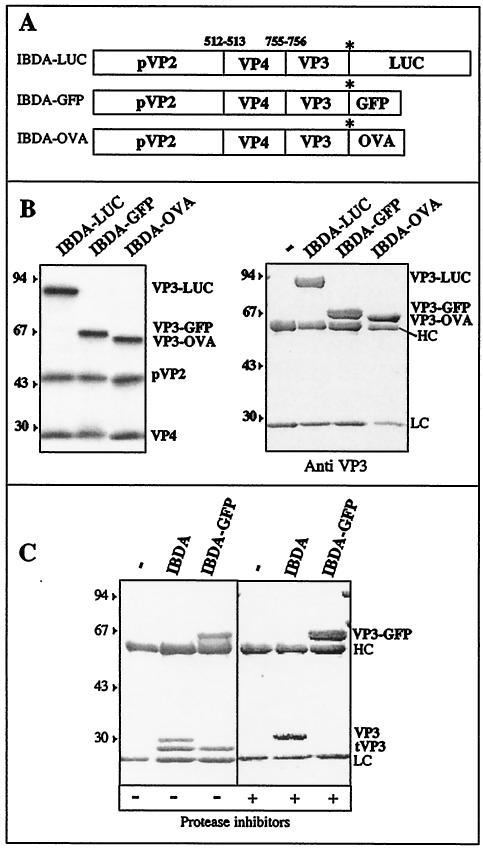
FIG. 1.
Construction of recombinant baculoviruses expressing the fusion polyproteins IBDA-LUC, IBDA-GFP, and IBDA-OVA. (A) Schematic representation of the three constructs expressing the IBDA polyproteins fused at their C terminus with luciferase (LUC), GFP, and ovalbumin (OVA). (B) Expression of the different chimerical polyproteins. (Left) The constructs were expressed in the rabbit reticulocyte expression system (Promega), and expression products were analyzed by SDS-PAGE. The different fusion proteins are cotranslationally processed to give rise to pVP2, VP4, and the VP3 proteins fused at their C terminus to luciferase, GFP, and ovalbumin. (Right) Immunoprecipitation analyses using an anti-VP3 antibody. Sf9 cells were infected with recombinant baculoviruses. Immune complexes were analyzed by SDS-PAGE under reducing conditions. The gel was stained with Coomassie blue. The relative molecular weights (shown in thousands) were determined by reference to marker proteins. HC and LC indicate the positions of the heavy and light chains of the immunoglobulins, respectively. Note the presence of a unique immunoprecipitated band in each lane, suggesting that no cleavage occurs inside the fusion proteins in infected cells incubated with an antiprotease inhibitor cocktail. (C) Protease susceptibility of VP3 and VP3-GFP visualized by SDS-PAGE. VP3 and VP3-GFP were expressed, extracted in the absence or presence of protease inhibitors, and immunoprecipitated. For each case, the first lane is the control immunoprecipitation without antigens.