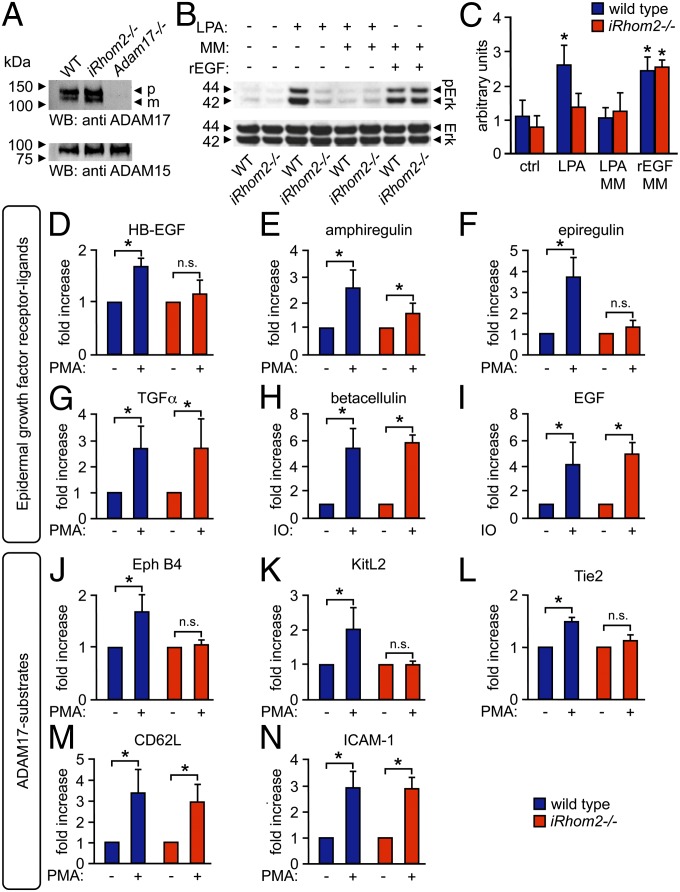
Fig. 1.
iRhom2 is required for metalloproteinase-dependent crosstalk between the LPA receptor and ERK1/2 and for the substrate selectivity of stimulated ADAM17-dependent shedding events. (A) Western blot analysis shows similar levels of mature ADAM17 in mEFs from iRhom2−/− mice and littermate controls (Adam17−/− mEFs are shown as negative control; ADAM15 Western blot is shown as loading control; n = 3). (B) WT and iRhom2−/− mEFs, starved for 24 h, were treated for 15 min with 10 µM LPA, 20 ng/mL recombinant EGF (rEGF), or medium alone. Where indicated, cells were pretreated for 20 min with 20 µM the hydroxamate metalloproteinase inhibitor MM. The Western blot was probed for phospho-ERK1/2, with total ERK1/2 as loading control. (C) Densitometric quantification of ERK1/2 phosphorylation relative to total ERK (n = 3). (D–N) WT and iRhom2−/− mEFs were transfected with the AP-tagged EGFR ligands HB-EGF (D), amphiregulin (E), epiregulin (F), TGFα (G), BTC (H), or EGF (I) or the ADAM17 substrates EphB4 (J), KitL2 (K), Tie2 (L), CD62L (M), or ICAM-1 (N). Shedding of all ADAM17 substrates was activated by treatment with 25 ng/mL PMA for 30 min (D–G and J–N), whereas shedding of the ADAM10 substrates BTC and EGF was activated by 2.5 µM ionomycin (IO) (H and I). *P ≤ 0.05; ±SEM (n = 3).