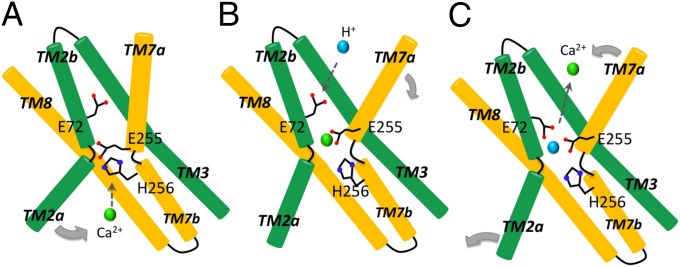
Fig. 6.
Proposed mechanism of Ca2+/H+ exchange by YfkE. (A) Inward open conformation stabilized by the protonated residue E255 for Ca2+ access. Conformational changes of the residues E255 and H256 may generate a path for Ca2+ binding and also shift TM2a for closure of the cytoplasmic entry. (B) Ca2+ binding at the oxygen umbrella leads to a rearrangement of the binding site, inducing conformational changes in TMs 7a and 6 (not shown for clarity) to transit the protein into an outward-facing conformation. (C) The residue E72 transfers H+ (blue sphere) from the periplasmic surface to the Ca2+-binding site. Ca2+ release is then triggered by protonation of E255 and/or H256, which may also reset the protein back to the inward-facing conformation.