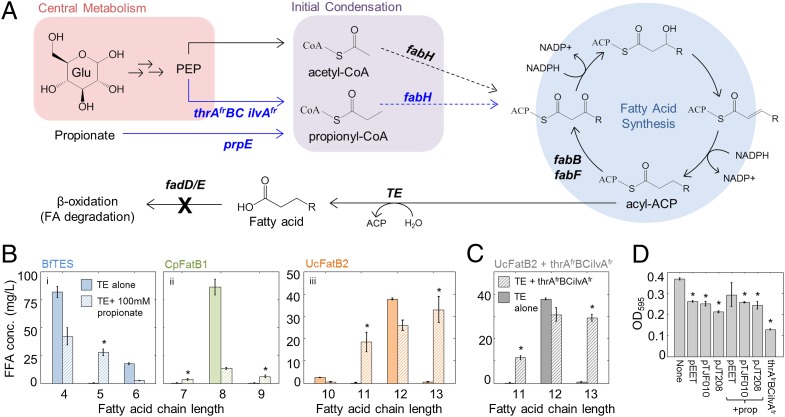
Fig. 1.
Engineering production of all even- and odd-length MCFAs in E. coli. (A) The KAS FabH elongates acetyl-CoA to form a four-carbon β-ketoacyl ACP, which is reduced to C4 acyl-ACP. In subsequent rounds of fatty acid synthesis, the KAS’s FabB and FabF elongate acyl-ACPs two carbons at a time to yield a range of even-chain–length fatty acyl-ACPs. Incorporation of propionyl-CoA in place of acetyl-CoA causes production of odd-chain acyl-ACPs; propionyl-CoA can be produced from propionate (prpE) or from expression of a genetic cassette that increases flux through the isoleucine pathway (thrAfrBC ilvAfr) (15). Acyl-ACPs can be hydrolyzed to FFAs by an appropriate thioesterase (TE). Fatty acid degradation can be blocked by knocking out the β-oxidation enzymes fadD or fadE. (B) GC-MS analysis of FFA production by strain S001 [BL21*(DE3) ∆fadD] containing plasmid pEET (BfTES) (i), pTJF010 (CpFatB1) (ii), or pJT208 (UcFatB2) (iii) in M9 +0.5% glycerol alone (solid bars) or supplemented with 100 mM propionate (striped bars) 24 h after IPTG induction (n = 3, error bars = SEM). FFAs shown for the no-propionate experiments accounted for 67% (i), 85% (ii) and 72% (iii) of total FFAs (full chain-length profiles in Fig. S1). FFAs shown for the propionate experiments accounted for (i) 79%, (ii) 65%, and (iii) 84% of the total (Fig. S1). (C) Production of odd-chain FFAs by S001-pJT208-pCOLAthrAfrBCilvAfr (n = 3, error bars = SEM). FFAs shown accounted for 81% of total. Asterisks in B and C indicate significantly increased odd-chain production (P < 0.05, one-tailed Student t test). (D) Final OD595 of strains in this figure, with or without propionate supplementation (n = 3, error bars = SEM). Asterisks indicate decreased OD595 compared with strain S001 alone (P < 0.05, one-tailed Student t test).