Fig. 3.
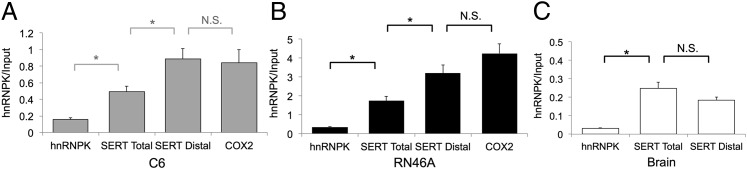
RNA immunoprecipitation confirms hnRNPK as a SERT distal polyadenylation element binding protein. Cell or tissue lysates were immunoprecipitated (IP) with an anti-hnRNPK antibody. Relative abundance of target sequences in hnRNPK immunoprecipitates (hnRNPK) was assessed by qPCR and compared with their abundance in total lysate (input) to determine the degree of hnRNPK interaction with the various target mRNA species. The hnRNPK and COX2 mRNA’s were quantified as negative and positive controls, respectively. (A) C6 cells. The proximal polyadenylation sequence of SERT present in all SERT mRNAs (SERT Total) is significantly enriched in hnRNPK IPs relative to the hnRNPK mRNA. SERT mRNAs containing the distal polyadenylation element (SERT Distal) are significantly more enriched in hnRNPK IPs than total SERT mRNA. COX2, a known hnRNPK-binding mRNA, is enriched to a similar degree as SERT distal. *P < 0.05, N.S., nonsignificant. Error bars, SEM. (B) RN46A cells. Total SERT mRNA is enriched in hnRNPK immunoprecipitates relative to hnRNPK and distal polyadenylation element-containing messages are significantly enriched relative to total SERT mRNA. *P < 0.05. Error bars, SEM. (C) Mouse brain homogenates. Both total and distal-containing SERT mRNAs are enriched relative to hnRNPK but are similar to one another. *P < 0.05, N.S., nonsignificant. Error bars, SEM.
