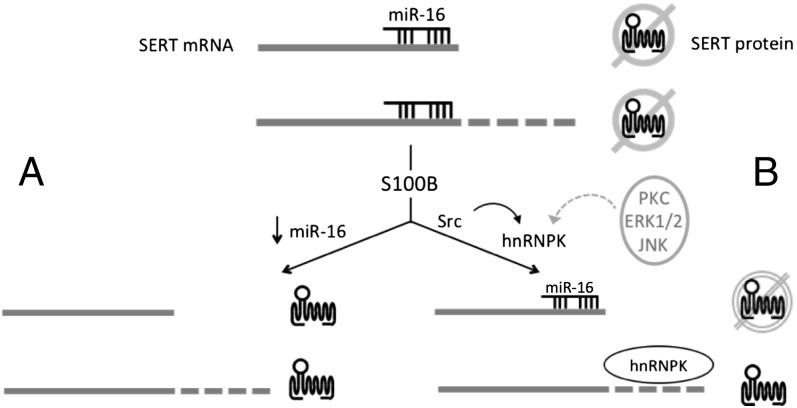
Fig. 7.
Diversity of translational regulation of SERT expression through alternative polyadenylation. Under basal conditions in cells expressing miR-16 translation of both proximal and distal polyadenylation forms of the SERT mRNA is blocked as indicated by slashed out topological model of SERT protein. (A) In some cell types (26, 27), S100B decreases expression of miR-16 derepressing translation of SERT protein from both proximal and distal polyadenylated mRNAs. (B) hnRNPK confers additional regulation of translation that our data suggests is specific to distal polyadenylated SERT mRNAs; however, we cannot rule out that hnRNPK may also regulate translation of proximal polyadenylated SERT mRNA as reflected in B by the dashed circle. S100B activates a Src-family kinase, which tyrosine phosphorylates hnRNPK, displacing miR-16 from distal polyadenylated messages and activating their translation. Additional signal transduction mechanisms can also modify hnRNPK function and may provide additional diversity of SERT translational regulation (dotted gray arrow).