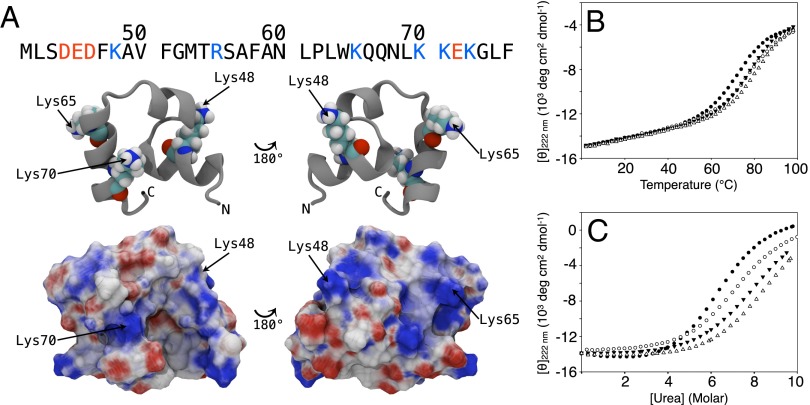
Fig. 1.
Mutation of solvent-exposed surface lysines stabilizes HP36. (A) The structure and sequence of HP36 showing the location of the lysine side chains. (B) Mutation of the surface lysines K48, K65, or K70 increases the melting temperature. Thermal unfolding curves are shown for WT HP36 (●), K48M (▼), K65M (□), and K70M (△). (C) Mutation of the surface lysines K48, K65, or K70 increases ΔG°unfolding. Urea-induced unfolding curves are shown for WT HP36 (●), K48M (▼), K65M (□), and K70M (△). Experiments were conducted at pH 5.0 in 10 mM sodium acetate and 150 mM sodium chloride.