Figure 5. Electrode contact formation mechanism.
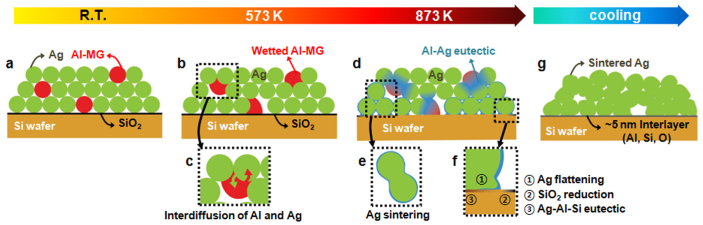
(a) Cross section of Al-MG/Ag paste printed on Si wafer. Green, red, yellow, and black represent silver, Al85Ni5Y8Co2 MG (Metallic Glass), Si wafer, and native SiO2 layer, respectively. (b) Al-MG particle spontaneously flows into the channel between the Ag particles during firing process. Since the contact angle between the MG and the Ag particles is below 90° and the viscosity of the MG is decreases in its SCL (supercooled liquid) region, the MG has a reasonably high driving force to fill the gap between Ag particles by the capillary effect. (c) Interdiffusion of Ag and Al occurs at the interface. (d) At the peak temperature (873 K) of the firing process, binary Al-Ag eutectic reaction occurs at the interface. Blue layer represents the Al-Ag eutectic liquid. (e) The Al-Ag eutectic liquid accelerates the sintering of Ag particles. (f) 1. Al-Ag eutectic liquid layer flattens the originally spherical Ag particle at the contact region with the Si wafer; 2. The native SiO2 layer on the Si wafer reduces to Si by Al in the Al-Ag eutectic liquid: 4/3Al + SiO2 → 2/3Al2O3 + Si; 3. Additional eutectic reactions of binary Al-Si (850 K) and ternary Ag-Al-Si (840 K) occur at the contact region with the Si substrate. (g) The presence of the eutectic liquid at the peak firing temperature leads to the formation of an ultra-thin interface layer between the Ag electrode and the Si wafer during cooling. Grey layer represents the ultra-thin interface layer.
