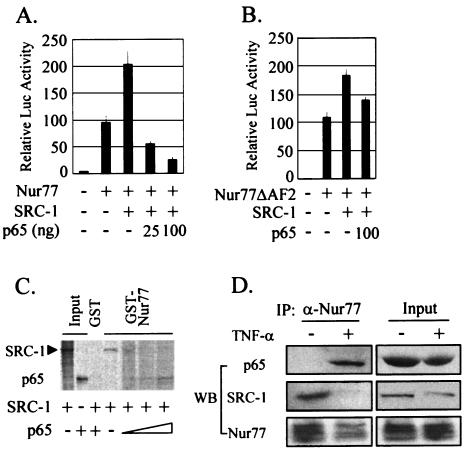
FIG. 8.
The p65 subunit of NF-κB competes with the Nur77 coactivator SRC-1 for Nur77 binding. (A) p65 coexpression blocked the SRC-1 enhancement of Nur77 transactivation. HeLa cells were cotransfected with (+) the indicated expression vectors (5 ng of Nur77, 25 ng of SRC-1, and 25 or 100 ng of p65) and 50 ng of the reporter NurRE-Luc. (B) Effect of SRC-1 on transactivation of Nur77ΔAF2, a deletion mutant of the AF2 domain. HeLa cells were cotransfected with the indicated expression vectors (5 ng of Nur77ΔAF2, 25 ng of SRC-1, and 100 ng of p65) and 50 ng of the reporter NurRE-Luc. (C) p65 interfered with the binding of the coactivator SRC-1 to Nur77 in GST pull-down assays. [35S]methionine-labeled SRC-1 was allowed to bind GST-Nur77 in the presence of increasing amounts of [35S]methionine-labeled p65. (D) p65 competed with SRC-1 for Nur77 binding in response to TNF-α in vivo. R2C cells were treated with TNF-α for 6 h and were harvested for coimmunoprecipitation (IP) experiments using anti-Nur77 antibody. Western blot (WB) analyses of the immunoprecipitates were performed using anti-p65 (α-p65), anti-SRC-1, and anti-Nur77 antibodies. The error bars indicate standard deviations.