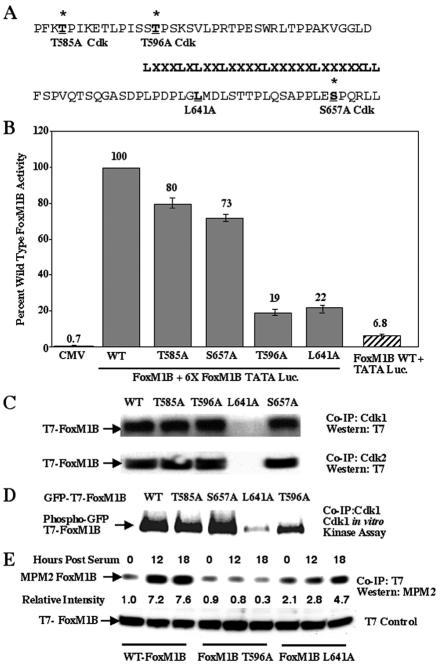
FIG. 3.
FoxM1B transcriptional activity requires association with Cdk1 protein and phosphorylation by Cdk1. (A and B) Mutation of the Cdk1 phosphorylation site at position 596 and Leu residue at position 641 causes diminished FoxM1B transcriptional activity. All of the Thr or Ser residues were changed to Ala, and the second Leu residue at position 641 in the LXL motif was changed to Ala (A). U2OS cells were transiently cotransfected with the reporter 6×-FoxM1B/FoxA-TATA-luciferase (49) and either CMV-empty, wild-type (WT) FoxM1B, FoxM1B T585A, FoxM1B T596A, FoxM1B S657A, or FoxM1B L641A. Twenty-four hours posttransfection, cells were harvested and processed for dual luciferase assays. The results are expressed as a percentage of the activity with wild-type FoxM1B, where CMV-empty served as a control for basal expression levels of the FoxM1B reporter gene. We also performed cotransfection controls with CMV FoxM1B wild-type and TATA-luciferase (Luc.) constructs, demonstrating that FoxM1B transcriptional activation requires the FoxM1B recognition sequence. Four separate transfection experiments were performed in triplicate to calculate standard deviations. (C) Mutation of the Leu 641 residue in the FoxM1B LXL motif diminishes FoxM1B protein association with either Cdk1 or Cdk2. U2OS cells were transiently transfected with either CMV T7-FoxM1B (lane 1), CMV T7-FoxM1B T585A (lane 2), CMV T7-FoxM1B T596A (lane 3), CMV T7-FoxM1B L641A (lane 4), or CMV T7-FoxM1B S657A (lane 5). U2OS cell lysates were prepared 48 h after transfection and coimmunoprecipitated (Co-IP) with either Cdk1 or Cdk2 polyclonal antibody. The coimmunoprecipitated proteins were subjected to Western blot analysis with a T7 epitope antibody. (D) Mutation of the Cdk1 phosphorylation site at position 596 decreases FoxM1B phosphorylation by Cdk1. U2OS cells were transiently transfected with CMV GFP-T7-FoxM1B (lane 1), CMV GFP-T7-FoxM1B T585A (lane 2), CMV GFP-T7-FoxM1B S657A (lane 3), CMV GFP-T7-FoxM1B L641A (lane 4), or CMV GFP-T7-FoxM1B T596A (lane 5). Transfected U2OS cell lysates were coimmunoprecipitated with polyclonal Cdk1 antibody and subjected to radioactive Cdk1 in vitro kinase assay and SDS-PAGE and autoradiography to visualize radioactively labeled proteins. Note that FoxM1B L641A mutant is not phosphorylated by Cdk1 because it no longer coimmunoprecipitatedwith the Cdk1 protein (panel C). (E) The FoxM1B T596A Cdk mutant protein shows diminished in vivo phosphorylation by Cdk protein. U2OS cells were transiently transfected with either CMV T7-FoxM1B, CMV T7-FoxM1B T596A, or FoxM1B L641A, transfected cells were serum starved for 48 h and either not stimulated or stimulated with serum for 12 or 18 h, and cells were harvested and protein extracts were prepared. Protein extracts were immunoprecipitated (IP) with T7 epitope antibody and then subjected to Western blot analysis with the MPM2 monoclonal antibody, which recognizes phosphorylated Cdk sites. Western blot analysis with T7 antibody demonstrated equal amounts of FoxM1B protein in all lanes. The relative intensity of the MPM2 signal was determined, and the FoxM1B level in cells not stimulated with serum was set at 1.