Figure 2.
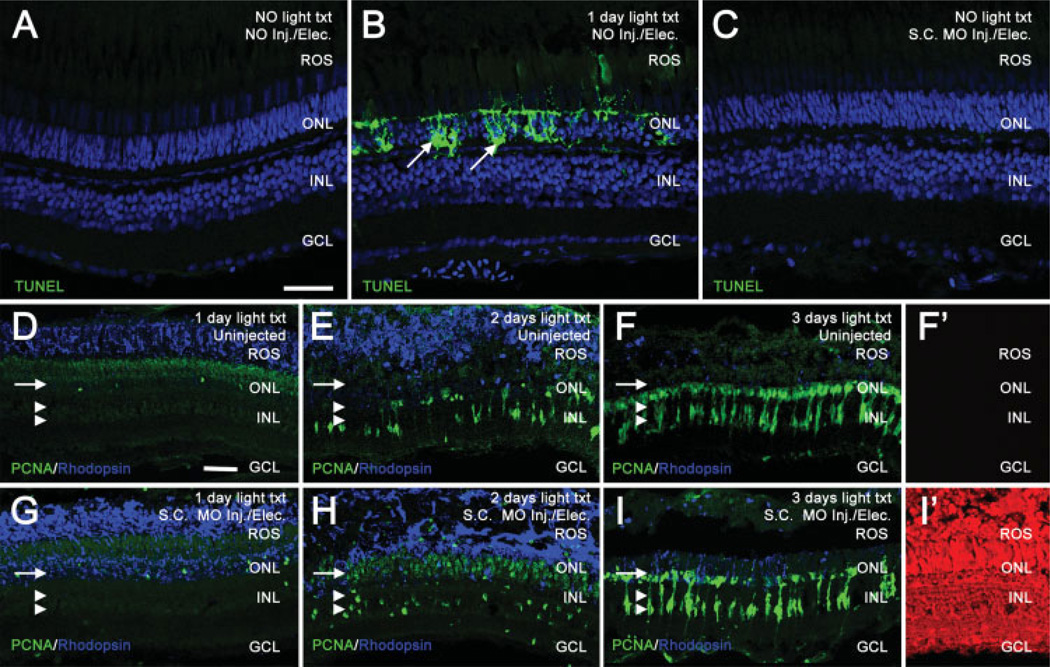
In vivo electroporation of morpholinos into the zebrafish retina does not cause cell death or adversely affect the regeneration response. TUNEL assays were used to compare cell death between uninjected, 24 h light-treated, and electroporated retinas (panels A–C). TO-PRO-3 (shown in blue) was used to stain nuclei. In uninjected, nonlight treated retinas, TUNEL labeling (shown in green) was not observed (panel A). In contrast, retinas light-treated for 24 h exhibited large amounts of TUNEL-positive nuclei in the ONL, representing dying photoreceptors (panel B, arrows). Non light-treated retinas electroporated with a Standard Control morpholino exhibited no detectable TUNEL labeling 24 h following electroporation (panel C), similar to uninjected control retinas. Uninjected retinas (panels D–F) and Standard Control electroporated retinas (panels G–I) were analyzed for PCNA (shown in green) and rhodopsin (shown in blue) immunolocalization. Panels D–F show the normal regenerative response at 1, 2, and 3 days of light, respectively. Rhodopsin decreases as rod photoreceptors degenerate. PCNA-positive, Müller glial derived, neuronal progenitors increase over the timecourse in both the INL (panels D–F, double arrowheads) and the ONL (panels D–F, arrow). Panel F′ shows background levels of red autofluorescence in uninjected retinas at 3 days of light treatment. Panels G–I show the normal regenerative response in retinas electroporated with a Standard Control morpholino. Similar to uninjected retinas, rhodopsin decreases and PCNA increases throughout the timecourse in both the INL (panels G–I, double arrowheads) and the ONL (panels G–I, arrow), indicating that electroporation does not adversely affect the regenerative response. Panel I′ shows a high level of lissamine-tagged Standard Control morpholino in all retinal layers at 3 days of light treatment. Scale bars: panel A, 25 µm (A–C); panel D, 25 µm (D–I′).