Abstract
Nitrogen (N) fertilizer is used routinely in potato (Solanum tuberosum) cultivation to maximize yield. However, it also affects sugar and free amino acid concentrations in potato tubers, and this has potential implications for food quality and safety because free amino acids and reducing sugars participate in the Maillard reaction during high-temperature cooking and processing. This results in the formation of color, aroma, and flavor compounds, but also some undesirable contaminants, including acrylamide, which forms when the amino acid that participates in the final stages of the reaction is asparagine. Another mineral, sulfur (S), also has profound effects on tuber composition. In this study, 13 varieties of potato were grown in a field trial in 2010 and treated with different combinations of N and S. Potatoes were analyzed immediately after harvest to show the effect of N and S fertilization on concentrations of free asparagine, other free amino acids, sugars, and acrylamide-forming potential. The study showed that N application can affect acrylamide-forming potential in potatoes but that the effect is type- (French fry, chipping, and boiling) and variety-dependent, with most varieties showing an increase in acrylamide formation in response to increased N but two showing a decrease. S application reduced glucose concentrations and mitigated the effect of high N application on the acrylamide-forming potential of some of the French fry-type potatoes.
Keywords: acrylamide, asparagine, free amino acids, potato, Solanum tuberosum, sugars
Introduction
Providing crops with adequate levels of nutrients ensures that the best yield possible is obtained, but attention is not always paid to the effect that plant nutrition has on crop composition and the consequences for food quality and safety. Inorganic nitrogen (N) is the mineral applied most often, usually in the form of ammonium nitrate or, less commonly in recent times, ammonium sulfate. The recommended N application rate for potato varies according to variety, soil type, and the nature of the previous crop, but in the United Kingdom (UK) is as high as 240 kg N/ha in some cases.1 N application promotes vegetative growth, delays tuber initiation and canopy senescence, and increases tuber size and yield. Another mineral that is applied to many crops is sulfur (S), but any benefit of S application to potatoes has not been established, and potatoes are usually grown without sulfur fertilization.
Both N and S application affect the protein composition of cereal grain,2 whereas N supply affects sugar concentrations and the interconversion of simple sugars and complex carbohydrates such as fructan in a variety of crop species.3 Both N and S supply also have potentially dramatic effects on the free amino acid concentrations of cereal grain.4−7 With respect to potato, De Wilde et al. showed that concentrations of sugars in tubers rose in response to N deprivation by up to 100% compared with adequately fertilized potatoes,8 and Kumar et al. similarly reported that plants adequately fertilized with N had lower reducing sugar concentration at harvest.9 Increased N fertilizer has also been shown to cause a rise in free amino acid concentrations,10 whereas S deprivation has been found to cause large increases in the concentrations of sugars.11 There were significant differences between the three varieties used in that study,11 but the concentration of glucose, for example, increased from 1.74 to 7.81 mmol/kg dry weight in tubers of variety Maris Piper. S deprivation also affected free amino acid concentrations, and this too was variety-dependent, with varieties King Edward and Maris Piper accumulating free glutamine in response to S deficiency whereas Prairie accumulated both free asparagine and free glutamine.
Another major management factor affecting the composition of potatoes is postharvest storage. Sugars, for example, accumulate rapidly in stored potato tubers in response to the temperature falling below approximately 8 °C (cold sweetening12), sprouting (dormancy break), and tuber senescence after long-term storage. Glucose and fructose may accumulate to such an extent that they cause localized blackening of chips (UK crisps) and French fries during frying. Tubers are therefore usually stored at 8–10 °C to prevent cold sweetening, whereas sprouting, which would occur at this temperature, is controlled by treatment with sprout suppressants such as chloropropham (CIPC).
Reducing sugars such as glucose and fructose react with free amino acids during high-temperature cooking (such as frying, baking, and roasting) and processing in a series of nonenzymic reactions given the umbrella name of the Maillard reaction.3,13,14 The Maillard reaction results in the formation of a plethora of products, many of which impart color, aroma, and flavor.3,13,14 However, it also gives rise to some undesirable contaminants, including acrylamide. Acrylamide forms when the amino acid that participates in the final stages of the reaction is asparagine,15−17 so free asparagine and reducing sugars can be regarded as its precursors, although other routes for its formation have been proposed.18−20 The relationship between the concentrations of these precursors in potato tubers and acrylamide formation during cooking and processing is complicated, with separate studies concluding reducing sugar concentration, free asparagine concentration, or free asparagine concentration as a proportion of the total free amino acid pool to be the determining factor.11,21−24 Recently, clear correlations have been shown between reducing sugar concentration and acrylamide-forming potential in nine varieties of potatoes grown commercially in the United Kingdom in 2009.25 Free asparagine and total free amino acid concentration also correlated significantly with acrylamide-forming potential in French fry but not chipping varieties, probably because French fry varieties contain higher concentrations of sugars. Another recent study modeled the kinetics of acrylamide formation in French fry production and concluded that both the fructose/glucose ratio and the ratio of asparagine to total free amino acids could affect acrylamide formation.26
Acrylamide has been classified as “probably carcinogenic to humans” by the World Health Organisation and the International Agency for Research on Cancer. The FAO/WHO Expert Committee on Food Additives has recommended that dietary exposure should be reduced, and the European Food Safety Authority (EFSA) issued “indicative” levels for acrylamide in food in early 2011.27 Fried potato products are major contributors to dietary exposure to acrylamide, and indicative levels have been set at 1000 ppb for chips and 600 ppb for French fries. The food industry has devised many strategies for reducing acrylamide formation by modifying food processing (compiled in a “Toolbox” produced by Food Drink Europe: http://www.fooddrinkeurope.eu/uploads/publications_documents/Toolboxfinal260911.pdf), and in Europe this has resulted in a significant downward trend for mean levels of acrylamide in potato chips from 763 ppb in 2002 to 358 ppb in 2011, a decrease of 53%.28 Nevertheless, processors remain vulnerable to fluctuations in the acrylamide-forming potential of the crop products that make up their raw material. Developing best practice for cultivation of particular crops, alongside variety selection and improvement, therefore, has an important part to play in acrylamide reduction strategies.29,30
Here we describe the analysis of 13 varieties of potatoes grown in a statistically designed field trial in 2010, treated with different combinations of N and S, and analyzed immediately after harvest. The aim of the study was to show the effect of N and S fertilization on concentrations of free asparagine, other free amino acids, sugars, and acrylamide-forming potential.
Materials and Methods
Chemicals
Chemicals were supplied as follows: nitram (ammonium nitrate) (GrowHow UK Ltd., Chester, UK); gypsum (calcium sulfate dihydrate) (Saint-Gobain, British Gypsum, Loughborough, UK); triple super phosphate ((CaH2PO4)2.H2O) and muriate of potash (KCl) (IAWS Fertilisers (UK) Ltd., Plymouth, UK); ethanol (95% v/v, analytical grade) (Thermo Fisher Scientific UK Ltd., Loughborough, UK); HCl (Corning Life Science; supplied by Sigma-Adrich Co. Ltd., Poole, UK); acrylamide-13C3 (Sigma-Adrich Co. Ltd.); KOH for IC chromatography (Thermo Fisher Scientific UK Ltd., Loughborough, UK); amino acid standards (Phenomenex, Torrence, CA, USA); isotopically labeled amino acids (Cambridge Isotope Laboratories, Inc., Andover, MA, USA); helium (high purity) (BOC Industrial Gases, Sheffield, UK).
Field Trial
The field trial was conducted at the Rothamsted farm site at Woburn, UK, in 2010. The design, which is shown in the Supporting Information, comprised three blocks (replicates), each containing 13 potato (Solanum tuberosum) varieties with combination treatments of nitrogen (N) (0, 100, or 200 kg/ha) and sulfur (S) (0, 15, or 40 kg/ha), making a total of nine different treatments. N was applied as ammonium nitrate and S as gypsum. Each of the nine main treatment plots was split, with five plants of each of the 13 varieties (split plots) within each main plot, giving a total of 45 plants per variety per block. All plots also received triple super phosphate at 128 kg/ha and muriate of potash at 458 kg/ha, and the trial was irrigated when required in the judgment of the farm manager. Meteorological data for the site from April to October 2010 are given in the Supporting Information. Tubers were harvested by hand between September 28 and October 28, 2010, according to whether they were early-, mid- or late-maturing varieties and to when canopy senescence was complete.
Free Amino Acid and Sugar Concentrations
Free amino acid and sugar concentrations were measured by an industry analytical laboratory (PepsiCo Europe, Beaumont Park, UK). In each case, tuber composite from the bulk sample derived from the five plants per treatment replicate was analyzed. For analysis of sugar concentrations, potato samples were cut and ground into pulp. An aliquot (42.5 g ± 0.1 g) was then blended for 2 min with 100 mL of deionized water and filtered through Whatman 2 V filter paper. A 5 mL volume of the filtered solution was quenched with 5 mL of ethanol and stored at −20 °C pending analysis. The quenched sample solution (0.2 mL) was diluted 50 times by mixing with 9.80 mL of Milli-Q water prior to analysis by ion chromatography with pulsed amperometric detection on a Dionex high-performance liquid chromatography (HPLC) instrument (ThermoFisher Scientific, Camberley, UK) with a Dionex CarboPac PA20 (3 × 150 mm) column, EC gold working electrode, and Ag/AgCl reference electrode. The instrument parameters were as follows: injection volume, 20 μL; flow rate, 0.37 mL/min; eluent mobile phase, 15 mM KOH; run time, 22 min; column and detector oven temperature, 25 °C.
Potato samples were also cut and ground into pulp for amino acid analyses. An aliquot (15.0 ± 0.1 g) was blended for 2 min at low speed with 60 mL of quench solution (ethanol/HCl 16:9 v/v) and filtered through Whatman 2 V filter paper, collecting approximately 5 mL of filtrate. A 0.1 mL aliquot of the filtrate was diluted to 1 mL with water and derivatized using the EZ-Faast amino acid derivatization kit for GC-MS (Phenomenex). The extraction procedure was modified to include the addition of a mixed internal standard solution of isotopically labeled amino acids to improve the accuracy and precision of the analysis. Gas chromatography–mass spectrometry (GC-MS) analysis of the derivatized sample was carried out on an Agilent GC-MS instrument (7890A GC system with a 5975C VL MSD mass spectrometer) with a Zebron amino acid (ZB-AAA) GC capillary column (10 m × 0.25 mm). The instrument parameters were as follows: split injection; injection volume, 2 μL with a 10:1 split ratio; inlet temperature, 250 °C; carrier gas, helium, at a constant flow rate of 1.1 mL/min; GC oven temperature gradient from 110 to 320 °C at 30 °C/min; ion source temperature, 240 °C; quadrupole temperature, 180 °C; transfer line, 280 °C; mass spectrometer scan range, m/z 45–450.
Fried Chips
Tubers were peeled and cut longitudinally to give tuber slices of 0.12–0.15 cm thickness. Samples (300 g fresh weight) were cooked for 3 min in high-oleic sunflower oil (Cargill, Lincoln, UK) at a starting temperature of 177 °C. Chip color was determined on the Hunter L, a, and b scales. Fried chip samples were graded in a light cabinet to remove any surface defects and then placed in a shallow dish. The surface was crushed flat before presentation to the viewing port of a Hunter DP-9000 colorimeter (Hunter Associates Laboratory Inc., Reston, VA, USA). The sample dish was turned through approximately 120° before a second reading was taken and again for a third reading. Samples did not leave the dish between readings. Moisture content was determined using a thermogravimetric analyzer (TGA) (LECO Instrument (UK) Ltd., Stockport, UK).
Acrylamide
Samples of fried chips were homogenized and aliquots extracted with a water solution containing acrylamide-13C3 as an internal standard. The solution was then purified by solid phase extraction with a proprietary sorbent phase followed by analysis by liquid chromatography–tandem mass spectrometry (LC-MS/MS).31 The method used was compatible with the Comité Européen de Normalization (European Committee for Standardisation) (CEN) standard method, which is due to be published under Mandate M463 (https://www.cen.eu/cen/Sectors/Sectors/Food/Documents/M_463.pdf).
Statistical Analyses
Analysis of variance (ANOVA) was used for each measured variable presenting biological replication, to assess the overall significance (F tests) of main effects and interactions between varieties nested within cooking type (French fry, chipping, or boiling) and fertilizer application (N and S). Means of interest were then compared using the least significant difference (LSD) at 5% based on the residual degrees of freedom (df) from the ANOVA. A natural log (to base e) transformation was used when necessary to account for some heterogeneity of variance, the residuals then conforming to the assumptions of ANOVA.
Results and Discussion
Potato Field Trials
Thirteen varieties of potato were grown in a randomized field trial at Woburn in Bedfordshire, UK, in 2010. The soil at this site is a sandy loam with very poor nutrient retention (soil sulfur (S) concentrations, for example, range from 0.5 to 1.8 mg/kg);32 crops grown there are therefore largely dependent on nutrients that are supplied through fertilization. The site has been used previously to investigate the effects of N and S nutrition on wheat and rye.4,33,34 The varieties studied comprised seven that are normally used for French fry production (Maris Piper, Pentland Dell, King Edward, Daisy, Markies, Russet Burbank, and Umatilla Russet), five that are normally used for chips (Lady Claire, Lady Rosetta, Saturna, Hermes, and Verdi), and one that is recommended predominantly for boiling but also for baking (Harmony).
The design of the field trial is shown in the Supporting Information. N was supplied at 0, 100, or 200 kg/ha (N0, N100, and N200) in combination with S at 0, 15, or 40 kg/ha (S0, S15, and S40), giving nine different combinations of N and S. The average yield of tubers from plants within each split plot was recorded and is available in the Supporting Information. ANOVA showed no significant effect of S application on yield, but did identify a significant (p = 0.026) variety by N interaction, indicating that N had a positive, variety-dependent effect on yield (Figure 1).
Figure 1.
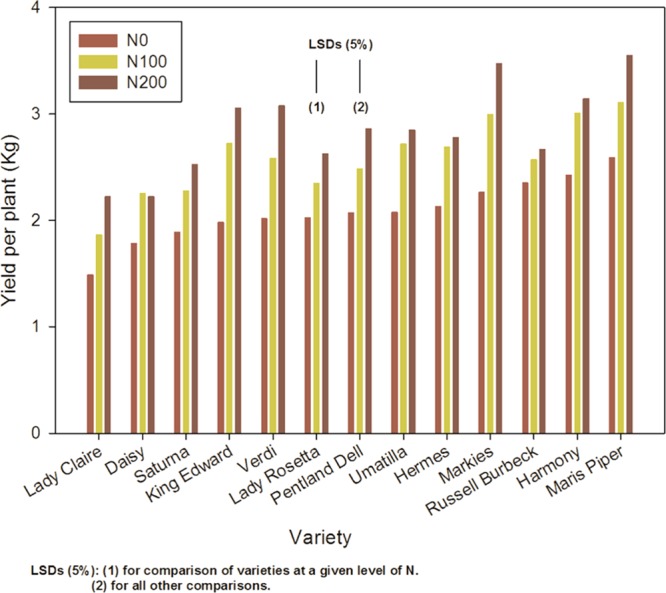
Yield per plant for 13 varieties of potato grown with three levels of nitrogen application, as indicated.
Free Amino Acid and Sugar Concentrations
Potatoes were harvested in September and October 2010 and analyzed by an industry analytical laboratory (PepsiCo Europe, Beaumont Park, UK). The analysis was performed on pulp produced by cutting and grinding tubers from the five plants per replicate within each treatment combination and blending them with deionized water. Free amino acid and sugar concentrations were determined analytically, whereas acrylamide formation was measured after potato slices had been fried to produce chips. Chip moisture, solids content, and color (Hunter L, a, and b) were also recorded. The data sets from these analyses, which are given in the Supporting Information, were subjected to analysis of variance (ANOVA). The p values arising from the analysis are shown in Table 1.
Table 1. p Values Denoting Significance of Main Effects and Interactions of Treatment Factors in ANOVA Analyses of Measured Variables for Potato Samplesa.
| amino acid | N | S | N.S | T | T.V | T.N | T.S | T.V.N | T.V.S | T.N.S | T.V.N.S |
|---|---|---|---|---|---|---|---|---|---|---|---|
| asparagine | <0.001 | 0.821 | 0.875 | 0.049 | <0.001 | 0.657 | 0.914 | <0.001 | 0.998 | 0.974 | 0.999 |
| glutamine | <0.001 | 0.846 | 0.661 | <0.001 | <0.001 | 0.031 | 0.554 | 0.004 | 0.894 | 0.365 | 0.992 |
| alanine | <0.001 | 0.194 | 0.323 | <0.001 | <0.001 | 0.442 | 0.841 | 0.002 | 0.324 | 0.357 | 0.048 |
| aspartate | 0.023 | 0.283 | 0.581 | 0.002 | <0.001 | <0.001 | <0.001 | 0.999 | 0.999 | <0.001 | 0.999 |
| glutamate | 0.150 | 0.181 | 0.272 | <0.001 | <0.001 | <0.001 | <0.001 | 0.999 | 0.999 | <0.001 | 0.999 |
| histidine | 0.434 | 0.434 | 0.498 | 0.493 | 0.423 | 0.587 | 0.588 | 0.433 | 0.433 | 0.684 | 0.437 |
| isoleucine | <0.001 | 0.200 | 0.662 | <0.001 | <0.001 | 0.032 | 0.898 | <0.001 | 0.041 | 0.109 | 0.473 |
| leucine | 0.087 | 0.994 | 0.999 | 0.838 | 0.069 | 0.950 | 0.547 | 0.026 | 0.838 | 0.631 | 0.922 |
| lysine | 0.003 | 0.549 | 0.976 | <0.001 | <0.001 | 0.282 | 0.335 | 0.026 | 0.199 | 0.763 | 0.938 |
| methionine | 0.006 | 0.913 | 0.953 | <0.001 | <0.001 | 0.102 | 0.918 | 0.018 | 0.132 | 0.999 | 0.981 |
| phenylalanine | <0.001 | 0.234 | 0.530 | <0.001 | <0.001 | 0.037 | 0.953 | <0.001 | 0.228 | 0.893 | 0.979 |
| proline | <0.001 | 0.124 | 0.916 | <0.001 | <0.001 | 0.231 | 0.428 | 0.019 | 0.349 | 0.978 | 0.911 |
| serine | <0.001 | 0.820 | 0.800 | <0.001 | <0.001 | 0.186 | 0.913 | 0.012 | 0.477 | 0.994 | 0.960 |
| threonine | 0.002 | 0.283 | 0.896 | <0.001 | <0.001 | 0.102 | 0.870 | <0.001 | <0.001 | 0.757 | 0.002 |
| tyrosine | 0.020 | 0.536 | 0.812 | 0.274 | <0.001 | 0.187 | 0.166 | <0.001 | 0.096 | 0.984 | 0.303 |
| valine | <0.001 | 0.993 | 0.700 | <0.001 | <0.001 | <0.001 | 0.012 | <0.001 | 0.930 | <0.001 | 0.779 |
| total | <0.001 | 0.670 | 0.396 | <0.001 | <0.001 | 0.005 | 0.106 | 0.175 | 0.165 | 0.003 | 0.852 |
| others not asparagine | <0.001 | 0.219 | 0.379 | <0.001 | <0.001 | 0.012 | 0.037 | 0.036 | 0.095 | <0.001 | 0.944 |
| acrylamide | 0.165 | 0.190 | 0.776 | <0.001 | <0.001 | 0.005 | 0.181 | 0.002 | 0.759 | 0.018 | 0.977 |
| fructose | 0.882 | 0.936 | 0.459 | <0.001 | <0.001 | 0.438 | 0.729 | 0.720 | 0.511 | 0.341 | 0.933 |
| glucose | 0.023 | 0.046 | 0.739 | <0.001 | <0.001 | 0.017 | 0.431 | 0.200 | 0.693 | 0.392 | 0.984 |
| sucrose | 0.289 | 0.393 | 0.958 | <0.001 | <0.001 | 0.266 | 0.361 | 0.943 | 0.935 | 0.591 | 0.998 |
| chip moisture (%) | 0.280 | 0.415 | 0.816 | <0.001 | <0.001 | 0.472 | 0.504 | 0.852 | 0.934 | 0.124 | 0.939 |
| solids (%) | 0.424 | 0.504 | 0.640 | <0.001 | <0.001 | 0.160 | 0.745 | 0.251 | 0.664 | 0.105 | 0.979 |
| Hunter a | 0.015 | 0.678 | 0.757 | <0.001 | <0.001 | 0.133 | 0.937 | 0.533 | 0.799 | 1.000 | 0.155 |
| Hunter b | 0.972 | 0.280 | 0.095 | 0.002 | <0.001 | 0.293 | 0.420 | 0.015 | 0.510 | 1.000 | 0.682 |
| Hunter L | 0.025 | 0.680 | 0.630 | 0.036 | 0.753 | 0.584 | 0.348 | 0.818 | 0.966 | 1.000 | 0.656 |
A dot indicates the interaction between any of the treatment factors, nitrogen (N), sulfur (S), type of potato (T), and variety (V), with variety being nested within type. p values in bold indicate the significant (p < 0.05, F test) ANOVA terms. Arginine could not be measured, whereas concentrations of glycine, ornithine, sarcosine, tryptophan, and cysteine were too low to be measured accurately.
Free asparagine concentration in the potatoes was significantly affected by N supply (p < 0.001), as were the concentrations of most of the free amino acids and total free amino acids. There was also a significant effect on free asparagine of type (p = 0.049), variety nested within type (French fry, chipping, or boiling) (p < 0.001), and an interaction of type, variety nested within type, and N (p < 0.001), showing that the two types and the varieties within each type responded differently to N supply. These interactions were also significant for total free amino acids, and there were additional effects of the interaction between type and nitrogen (p = 0.005). All of the sugars showed significant (p < 0.001) effects of type and variety nested within type, but glucose also responded to N (p = 0.023) and to the interaction between type and N (p = 0.017).
S feeding has been shown previously to cause a decrease in free asparagine and reducing sugar concentrations in potatoes grown in pots under glass.11,24 Sulfur deficiency also causes dramatic increases in free asparagine and total free amino acid concentrations in wheat grain,4,6,7 but not in rye, at least under field conditions.34,35 In this field-based study, the application of S fertilizer had no significant effect on free asparagine concentration in the potatoes, although it did have some significant effects in combination with the other variables (N, type, and variety) on aspartate, glutamate, threonine, and valine concentrations (Table 1). It is possible that the level of sulfur deficiency required to induce the effects on asparagine observed in the pot-based experiments were not achieved in the field or that free amino acid concentrations in potatoes are affected greatly if the plants are grown in pots under glass. However, the application of S fertilizer in this study did have a significant effect on glucose concentration (p = 0.046), with increasing S related to decreasing glucose.
These effects were investigated further, and the relevant means tables for the free amino acid and sugar concentrations are presented in Tables 2. Table 2a gives the means on the natural log (to base e) scale showing the effect of variety nested within type for total free amino acids and the two reducing sugars, glucose and fructose, in the potatoes. The back-transformed means are also given and for total free amino acids range from 47.4 mmol/kg for Daisy to 174.3 mmol/kg for Russet Burbank. The back-transformed means for glucose concentration ranged from 0.05 g/kg in Verdi to 2.52 g/kg in Russet Burbank and 6.03 g/kg in the boiling variety, Harmony (the concentration in Harmony was therefore >120 times higher than that in Verdi). Note that the back-transformed means for glucose were lower than raw means because of the variability in the data on the raw scale. This was particularly so for Harmony, but also to some extent for King Edward and Verdi. The raw data mean for Verdi, for example, was 0.186 g/kg, compared with the back-transformed mean of 0.05 g/kg.
Table 2a. Variety Nested within Type Means (n = 27) Tables for Total Free Amino Acids, Glucose, and Fructose on the Natural Log (to Base e) Scale and for Sucrose on the Raw Scale, Following ANOVA Analyses, for Potato Samplesa.
| type | variety | total free amino acids (mmol/kg) | glucose (g/kg) | fructose (g/kg) | sucrose (g/kg) |
|---|---|---|---|---|---|
| chipping | Hermes | 5.058 (157.3) | –2.408 (0.09) | –1.778 (0.17) | 5.70 |
| Lady Claire | 4.416 (82.8) | –1.921 (0.15) | –2.874 (0.06) | 5.94 | |
| Lady Rosetta | 4.288 (72.8) | –2.412 (0.09) | –2.064 (0.13) | 4.52 | |
| Saturna | 4.654 (105.0) | –2.152 (0.12) | –1.560 (0.21) | 4.70 | |
| Verdi | 4.387 (80.4) | –2.989 (0.05) | –1.183 (0.31) | 6.27 | |
| French fry | Daisy | 3.859 (47.4) | –0.733 (0.48) | –1.250 (0.29) | 8.55 |
| King Edward | 5.120 (167.3) | 0.119 (1.13) | –0.485 (0.62) | 9.82 | |
| Maris Piper | 4.851 (127.9) | –1.242 (0.29) | –0.990 (0.37) | 8.62 | |
| Markies | 4.787 (119.9) | –1.133 (0.32) | –1.857 (0.16) | 9.19 | |
| Pentland Dell | 5.134 (169.7) | –0.393 (0.68) | 0.270 (1.31) | 5.83 | |
| Russet Burbank | 5.161 (174.3) | 0.924 (2.52) | 0.453 (1.57) | 9.05 | |
| Umatilla Russet | 4.962 (142.8) | 0.445 (1.56) | –1.577 (0.21) | 9.94 | |
| boiling | Harmony | 4.553 (94.9) | 1.796 (6.03) | –1.165 (0.31) | 8.30 |
| SED (df) | 0.0705 (208) | 0.3491 (209) | 0.2074 (212) | 0.779 (213) | |
| LSD (5%) | 0.1390 | 0.6882 | 0.4089 | 1.536 | |
The standard error of the difference (SED), degrees of freedom (df), and least significant difference (LSD) values at the 5% level of significance for comparison of means on the log scale are also included. Back-transformed means are given in parentheses.
Surprisingly, the back-transformed mean for fructose concentration in Harmony was much lower (0.31 g/kg), whereas it ranged from 0.06 g/kg in Lady Claire to 1.57 g/kg in Russet Burbank, a 26-fold difference. Sucrose concentrations are given on the raw scale in the table and were much more consistent across the varieties, ranging from 4.52 g/kg in Lady Rosetta to 9.94 g/kg in Umatilla Russet. Sucrose was the most abundant sugar in all 13 varieties.
The means showing the effect of the interaction between N supply and type on glucose concentration in the potatoes are given in Table 2b. In the chipping type, glucose concentration was slightly lower when N was supplied at 100 kg/ha (N100) than when N was not supplied (N0) (back-transformed mean of 0.07 g/kg compared with 0.09 g/kg) but then increased significantly at N200 (back-transformed mean = 0.13 g/kg). The French fry varieties, on the other hand, showed a bigger and statistically significant reduction in concentration from N0 to N100, whereas the concentration at N200 was similar to that at N0. The boiling type, Harmony, was different again, with the back-transformed mean for glucose concentration rising from 2.66 g/kg at N0 to 14.69 g/kg at N100 (a >5-fold increase) and 5.60 g/kg at N200. Two previous studies have reported that lack of N causes an increase in glucose concentration in potatoes.8,9 The data reported here show a more complicated picture, with N having type- and variety-specific effects on glucose concentrations.
Table 2b. Type by Nitrogen (N) Means Table for Glucose on the Natural Log (to Base e) Scale following ANOVA Analyses, for Potato Samples, with Replication, na.
| type | N (kg/ha) | glucose (g/kg) |
|---|---|---|
| chipping (n = 45) | 0 | –2.387 (0.09) |
| 100 | –2.676 (0.07) | |
| 200 | –2.065 (0.13) | |
| French Fry (n = 63) | 0 | –0.140 (0.87) |
| 100 | –0.544 (0.58) | |
| 200 | –0.179 (0.84) | |
| boiling (n = 9) | 0 | 0.979 (2.66) |
| 100 | 2.687 (14.69) | |
| 200 | 1.722 (5.60) | |
| SED (209 df), LSD for means with same N level | ||
| n = 9 and n = 45 | 0.4684, 0.9234 | |
| n = 9 and n = 63 | 0.4571, 0.9011 | |
| n = 45 and n = 63 | 0.2504, 0.4936 | |
| SED (df), LSD for other comparisons | ||
| n = 9 and n = 9 | 0.5922 (221), 1.1671 | |
| n = 45 and n = 45 | 0.2286 (224), 0.4506 | |
| n = 63 and n = 63 | 0.1932 (101), 0.3833 | |
The standard error of the difference (SED) and the least significant difference (LSD) values at the 5% level of significance for comparison of means on the log scale are also included. Back-transformed means are given in parentheses.
Sulfur had a direct effect on glucose concentration, independent of the other variables, as shown in Table 2c. Sulfur fertilization brought about a reduction in the back-transformed mean for glucose concentration from 0.46 g/kg at S0 to 0.39 g/kg at S15 and 0.34 g/kg at S40, the reduction from S0 to S40 being 26%. This is the first report of an effect of sulfur on sugar concentration in potato.
Table 2c. Sulfur (S) Means (n = 117) Table for Glucose (Milligrams per Kilogram) on the Natural Log (to Base e) Scale following ANOVA Analyses, for Potato Samplesa.
| S |
|||
|---|---|---|---|
| 0 kg/ha | 15 kg/ha | 40 kg/ha | SED, LSD (5%) (16 df) |
| –0.770 (0.46) | –0.937 (0.39) | –1.085 (0.34) | 0.1151, 0.2439 |
The standard error of the difference (SED) and the least significant difference (LSD) values at the 5% level of significance for comparison of means on the log scale are also included. Back-transformed means are given in parentheses.
The means for the interaction between variety nested within type and N on free asparagine and glutamine concentrations in the potatoes are given in Table 2d. There was a trend for substantial increases in free asparagine accumulation in all of the varieties in response to N fertilization, but the underlying variance meant that it was statistically significant (at 5%, using the LSD) only for Verdi, which increased from 25.3 mmol/kg at N0 to 61.7 mmol/kg at N200; Daisy, which increased from 0.23 mmol/kg at N0 to 10.9 mmol/kg at N200; and Harmony, for which the back-transformed mean increased from 25.4 mmol/kg at N0 to 64.3 mmol/kg at N200. Note that the back-transformed mean for Daisy at N0 was low due to three observations at the limit of detection, and the raw data mean was 5.32 mmol/kg. However, this concentration was still very low compared with the other varieties and only just over half that for Daisy at N200. There was also a trend for substantial increases in free glutamine (p = 0.004 for the variety nested within type by N (T.V.N) interaction; Table 1), and the relevant means for this amino acid are also shown in Table 2d. The increases were statistically significant (at 5%, using the LSD) for Harmony, Russet Burbank, Markies, Verdi, Lady Rosetta, Lady Claire, and Hermes. In all of these varieties, the back-transformed mean for glutamine concentration at N200 was more than double that at N0, but was still substantially lower than that for asparagine. In Daisy, on the other hand, glutamine concentration (back-transformed mean ranging from 9.8 mmol/kg at N0 to 14.0 mmol/kg at N200) was higher than asparagine concentration, whereas in King Edward, glutamine and asparagine concentrations were very similar, particularly at high N (back-transformed mean = 31.7 mmol/kg at N0 and 47.4 mmol/kg at N200 compared with 39.5 and 49.1 mmol/kg). This is important because glutamine could compete with asparagine for participation in the Maillard reaction.36
Table 2d. Variety Nested within Type by Nitrogen (N) Means (n = 9) Table for Free Asparagine and Glutamine on the Natural Log (to Base e) Scale following ANOVA Analyses, for Potato Samplesa.
| asparagine
(mmol/kg) |
glutamine
(mmol/kg) |
||||||
|---|---|---|---|---|---|---|---|
| type | variety | N0 | N100 | N200 | N0 | N100 | N200 |
| chipping | Hermes | 4.148 (63.3) | 4.296 (73.4) | 4.613 (100.8) | 2.635 (13.9) | 2.857 (17.4) | 3.378 (29.3) |
| Lady Claire | 2.974 (19.6) | 3.686 (39.9) | 3.700 (40.4) | 2.256 (9.5) | 3.033 (20.8) | 3.203 (24.6) | |
| Lady Rosetta | 3.136 (23.0) | 3.61 (37.0) | 3.677 (39.5) | –0.284 (0.8) | 2.277 (9.7) | 2.357 (10.6) | |
| Saturna | 3.816 (45.4) | 3.974 (53.2) | 4.270 (71.5) | 2.215 (9.2) | 2.413 (11.2) | 2.771 (16.0) | |
| Verdi | 3.231 (25.3) | 3.665 (39.1) | 4.122 (61.7) | 1.753 (5.8) | 2.162 (8.7) | 2.645 (14.1) | |
| French fry | Daisy | –1.449 (0.23b) | 2.264 (9.62) | 2.385 (10.9) | 2.282 (9.8) | 2.614 (13.7) | 2.637 (14.0) |
| King Edward | 3.676 (39.5) | 3.967 (52.8) | 3.894 (49.1) | 3.456 (31.7) | 3.881 (48.5) | 3.858 (47.4) | |
| Maris Piper | 3.491 (32.8) | 3.652 (38.6) | 3.776 (43.6) | 2.817 (16.7) | 3.009 (20.3) | 3.244 (25.6) | |
| Markies | 3.545 (34.6) | 3.683 (39.8) | 3.935 (51.2) | 2.612 (13.6) | 3.020 (20.5) | 3.358 (28.7) | |
| Pentland Dell | 3.963 (52.6) | 4.272 (71.7) | 4.244 (69.7) | 3.065 (21.4) | 3.298 (27.1) | 3.424 (30.7) | |
| Russet Burbank | 4.004 (54.8) | 4.345 (77.1) | 4.605 (100.0) | 2.946 (19.0) | 3.539 (34.4) | 3.786 (44.1) | |
| Umatilla Russet | 3.973 (53.1) | 4.132 (62.3) | 4.389 (80.6) | 3.003 (20.1) | 3.284 (26.7) | 3.618 (37.3) | |
| boiling | Harmony | 3.235 (25.4) | 3.835 (46.3) | 4.163 (64.3) | 2.621 (13.7) | 3.142 (23.1) | 3.504 (33.2) |
| SED, LSD (5%), same N | 0.4060, 0.8004 (208 df) | 0.3393, 0.6689 (208 df) | |||||
| SED, LSD (5%), all others | 0.4012, 0.7906 (223 df) | 0.3393, 0.6686 (223 df) | |||||
The standard error of the difference (SED) and the least significant difference (LSD) values at the 5% level of significance for comparison of means on the log scale are also included. Back-transformed means are given in parentheses.
Back-transformed mean is low due to three observations at the limit of detection; raw data mean was 5.32.
Finally, the means showing the effect of the interaction between type, N, and S on total free amino acid concentration are given in Table 2e. Total free amino acid concentration rose significantly with increased N in all types. S, on the other hand, had no significant effect in the French fry types and an inconsistent effect on the other types, the concentration in Harmony at N0 S15, for example, being much lower than that at N0 S0 or N0 S40, and the concentration in the chipping type being lower at N0 S40 than at N0 S0 but higher at N100 S40 than at N100 S0.
Table 2e. Type by Nitrogen (N) by Sulfur (S) Means for Total Free Amino Acids (Millimoles per Kilogram) on the Natural Log (to Base e) Scale following ANOVA Analyses, for Potato Samples, with Replication, na.
| S |
||||
|---|---|---|---|---|
| type | N (kg/ha) | 0 kg/ha | 15 kg/ha | 40 kg/ha |
| chipping (n = 15) | 0 | 4.374 (79.4) | 4.319 (75.1) | 4.169 (64.6) |
| 100 | 4.523 (92.1) | 4.569 (96.4) | 4.705 (110.5) | |
| 200 | 4.811 (122.9) | 4.802 (121.8) | 4.773 (118.3) | |
| French fry (n = 21) | 0 | 4.594 (98.9) | 4.631 (102.6) | 4.677 (107.4) |
| 100 | 4.871 (130.5) | 4.941 (139.9) | 4.867 (129.9) | |
| 200 | 4.956 (142.0) | 4.995 (147.7) | 5.021 (151.6) | |
| boiling (n = 3) | 0 | 4.267 (71.3) | 3.539 (34.4) | 4.471 (87.4) |
| 100 | 4.589 (98.4) | 4.590 (98.5) | 4.756 (116.3) | |
| 200 | 4.973 (144.5) | 4.997 (148.0) | 4.798 (121.3) | |
| SED (208 df), LSD for means with same levels of N and S | ||||
| n = 3 andn = 15 | 0.1638, 0.3230 | |||
| n = 3 andn = 21 | 0.1599, 0.3153 | |||
| n = 15 and n = 21 | 0.0876, 0.1727 | |||
| SED (df), LSD for other comparisons | ||||
| n = 3 andn = 3 | 0.2082 (221), 0.4103 | |||
| n = 15 and n = 15 | 0.0931 (223), 0.1835 | |||
| n = 21 and n = 21 | 0.0706 (82), 0.1404 | |||
The standard error of the difference (SED) and the least significant difference (LSD) values at the 5% level of significance for comparison of means on the log scale are also included. Back-transformed means are given in parentheses.
Acrylamide Formation in Chips
Acrylamide formation in chips made from the potatoes was measured in the N0 S0, N200 S0, N0 S15, and N200 S15 samples. This subset was selected to include the two nitrogen extremes and the current commercially relevant range of sulfur. The analysis showed significant (p < 0.001) effects of type and variety nested within type (Table 1), consistent with a previous report that potato types and varieties within types differ significantly in acrylamide-forming potential.25 Whereas the main effect of neither N nor S was significant, there were significant effects of the interaction between N and type (p = 0.005), N, typ,e and variety nested within type (p = 0.002), and type, N, and S (p = 0.018). The relevant means are given in Tables 3a and 3b. The biggest effect of N was on Harmony, with an increase in the back-transformed mean for acrylamide formation from 2576 ppb at N0 to 6336 ppb at N200. All but one of the French fry varieties also showed an increase, the largest being in Russet Burbank, for which the back-transformed mean rose from 2103 ppb at N0 to 2884 ppb at N200, an increase of 37%. Pentland Dell bucked the trend, showing a decrease from 1183 ppb at N0 to 499 ppb at N200, a fall of 58%. There was no consistent effect of N on the chipping varieties, with the back-transformed mean for Saturna showing a decrease from 291 ppb at N0 to 124 ppb at N200, a fall of 57%, whereas Hermes showed a small decrease, Lady Claire was almost unchanged, and Lady Rosetta and Verdi increased slightly.
Table 3a. Type by Variety (Nested within Type) by Nitrogen (N) Means (n = 6) for Chip Acrylamide (Parts per Billion) on the Natural Log (to Base e) Scale following ANOVA Analysesa.
| N |
|||
|---|---|---|---|
| type | variety | 0 kg/ha | 200 kg/ha |
| chipping | Hermes | 5.744 (312) | 5.657 (286) |
| Lady Claire | 5.861 (351) | 5.846 (346) | |
| Lady Rosetta | 4.895 (133) | 5.138 (170) | |
| Saturna | 5.676 (291) | 4.822 (124) | |
| Verdi | 5.451 (232) | 5.763 (318) | |
| French fry | Daisy | 5.725 (306) | 6.312 (551) |
| King Edward | 6.485 (655) | 6.601 (736) | |
| Maris Piper | 6.040 (419) | 6.183 (484) | |
| Markies | 6.030 (416) | 6.374 (586) | |
| Pentland Dell | 7.076 (1183) | 6.212 (499) | |
| Russet Burbank | 7.651 (2103) | 7.967 (2884) | |
| Umatilla Russet | 6.883 (976) | 7.053 (1156) | |
| boiling | Harmony | 7.854 (2576) | 8.754 (6336) |
| SED (df), LSD (5%) for means with the same N | 0.2645 (83), 0.5261 | ||
| SED (df), LSD (5%) for other comparisons | 0.2620 (88), 0.5206 | ||
Note that combinations with 100 kg/ha N (and 40 kg/ha S) were not assayed. The standard error of the difference (SED) and the least significant difference (LSD) values at the 5% level of significance for comparison of means on the log scale are also included. Back-transformed means are given in parentheses.
Table 3b. Type by Nitrogen (N) by Sulfur (S) Means for Chip Acrylamide (Parts per Billion) on the Natural Log (to Base e) Scale following ANOVA Analyses, with Replication, na.
| S |
|||
|---|---|---|---|
| type | N (kg/ha) | 0 kg/ha | 15 kg/ha |
| chipping | 0 | 5.630 (279), n = 15 | 5.421 (226), n = 15 |
| 200 | 5.313 (203), n = 15 | 5.577 (264), n = 15 | |
| French fry | 0 | 6.578 (719), n = 21 | 6.533 (687), n = 21 |
| 200 | 6.831 (926), n = 21 | 6.513 (674), n = 21 | |
| boiling | 0 | 7.912 (2730), n = 3 | 7.795 (2428), n = 3 |
| 200 | 8.791 (6575), n = 3 | 8.716 (6100), n = 3 | |
| SED (83 df), LSD for means with same levels of N and S | |||
| n = 3 and n = 15 | 0.2898, 0.5761 | ||
| n = 3 and n = 21 | 0.2828, 0.5625 | ||
| n = 15 and n = 21 | 0.1549, 0.3079 | ||
| SED (df), LSD for other comparisons | |||
| n = 3 and n = 3 | 0.3705 (88), 0.7363 | ||
| n = 15 and n = 15 | 0.1558 (87), 0.3098 | ||
| n = 21 and n = 21 | 0.1317 (25), 0.2712 | ||
Note that combinations with 100 kg/ha N and 40 kg/ha S were not assayed. The standard error of the difference (SED) and the least significant difference (LSD) values at the 5% level of significance for comparison of means on the log scale are also included. Back-transformed means are given in parentheses.
The effect of high N was mitigated to some extent in the French fry varieties by the application of sulfur, resulting in a significant (p < 0.018) type by N by S interaction. The relevant means are given in Table 3b. At S0, the back-transformed mean acrylamide level for the French fry varieties was 719 ppb, rising to 926 ppb at N200. This rise did not occur when S was applied, resulting in a back-transformed mean at N200 S15 of only 674 ppb, 27% lower than the N200 S0 level. In contrast, the overall back-transformed mean for the chipping varieties at N200 was not significantly different from that at N0, regardless of sulfur application, whereas that for the boiling type, Harmony, showed a big increase from 2730 ppb at N0 S0 to 6575 at N200 S0, but this was not reduced significantly (at 5% using the LSD) by the application of sulfur.
N application also had a significant effect on chip color (Hunter L, a, and b), on its own (L and a) or interacting with variety nested within type (b) (see p values in Table 1). Independent of N effects, Hunter L and a were also affected significantly by type and Hunter a and b by variety nested within type.
Implications for Commercial Potato Cultivation
The study was conducted over a single growing season, and the potential effects of different environmental conditions from one season to another should be considered in the assessment of the implications of the study for commercial potato cultivation. Furthermore, acrylamide formation was measured in chips as a standard method, whereas some of the varieties studied would not normally be used for chip production. Nevertheless, some important conclusions can be drawn. The study showed that N application can affect acrylamide-forming potential in potatoes but that the effect is type (French fry versus chipping) dependent, with French fry varieties showing an effect that is not apparent in chipping varieties. The different varieties within type also showed different responses, with most varieties showing an increase in acrylamide formation in N200 compared with N0 but Pentland Dell and Saturna showing a large decrease, making the situation even more complicated. A previous study21 reported no effect of N on acrylamide-forming potential, but that study did not consider French fry and chipping types separately. That study also reported that reducing sugar concentrations correlated with acrylamide formation, but no significant correlation was found for free asparagine or total free amino acids. In contrast, we have shown previously that free asparagine concentration correlates with acrylamide-forming potential specifically in French fry varieties.25
Currently, advice on N application rates does not take acrylamide-forming potential into account. N application, of course, also affects plant health and yield, and a balance between achieving the best possible yield while not exacerbating acrylamide-forming potential may be difficult to achieve in some cases. However, it was notable that variety Russet Burbank, for example, showed the largest difference in acrylamide formation (37%) of all the French fry varieties when grown with low or high levels of N but showed a relatively small difference in yield, at least in this single-year study. The current recommendation for N application for this and other main crop varieties in low N soils in the United Kingdom is up to 180 kg/ha.1 The recommendation for Harmony, Lady Claire, and Lady Rosetta is higher, at up to 220 kg/ha, whereas that for Markies is lower at up to 140 kg/ha.
Sulfur fertilizer is not usually applied to potatoes but was included in the study because S feeding had been shown previously to affect the composition of potatoes grown in pots under glass.11 Importantly, S application in this study was shown to reduce glucose concentrations and, possibly as a result of this, to mitigate the effect of high N application on the acrylamide-forming potential of some of the French fry-type potatoes. However, S application had no significant effect on yield, and it would be difficult to convince farmers that expenditure on S fertilizer represented good value for money. Nevertheless, the mitigating effect of S on acrylamide formation in chips produced from the French fry-type potatoes was 27%, and in chips produced from variety Daisy, for example, the acrylamide level was 407 ppb at N200 S15 compared with 877 ppb at N200 S0. As with N, advice on S application would have to be carefully tailored for specific varieties.
The study included a variety, Harmony, that is generally used for processes in which acrylamide would not form, although it is sometimes marketed as a baking potato. This variety showed very high levels of acrylamide formation, up to 6336 ppb in chips, highlighting the need to exclude inappropriate varieties from the raw material used for processes in which acrylamide may form. The 6336 ppb level in chips was obtained with potatoes grown at N200, whereas at N0 the level was 2576 ppb, 59% lower, demonstrating a very clear link between N application and acrylamide-forming potential in this variety.
Supporting Information Available
Data showing yield, free amino acid and sugar concentrations, acrylamide formed in chips, chip color (Hunter L and a), the design of the field trial, and meteorological data. This material is available free of charge via the Internet at http://pubs.acs.org.
The study was financially supported by the Biotechnology and Biological Sciences Research Council (BBSRC) of the United Kingdom and industry partners through the Sustainable Arable LINK program “Producing Low Acrylamide Risk Potatoes” (http://www.acrylamide-potato.org.uk/). Rothamsted Research receives grant-aided support from the BBSRC.
The authors declare no competing financial interest.
Supplementary Material
References
- Sinclair A.; Morrice L.; Wake S.; Booth E.. Technical Note TN625: Nitrogen Recommendations for Cereals, Oilseed Rape and Potatoes; Scottish Agricultural College: Aberdeen, Scotland, 2009. [Google Scholar]
- Shewry P. R.; Tatham A. S.; Halford N. G. Nutritional control of storage protein synthesis in developing grain of wheat and barley. Plant Growth Regul. 2001, 34, 105–111. [Google Scholar]
- Halford N. G.; Curtis T. Y.; Muttucumaru N.; Postles J.; Mottram D. S. Sugars in crop plants. Ann. Appl. Biol. 2011, 158, 1–25. [Google Scholar]
- Muttucumaru N.; Halford N. G.; Elmore J. S.; Dodson A. T.; Parry M. A. J.; Shewry P. R.; Mottram D. S. The formation of high levels of acrylamide during the processing of flour derived from sulfate-deprived wheat. J. Agric. Food Chem. 2006, 54, 8951–8955. [DOI] [PubMed] [Google Scholar]
- Lea P. J.; Sodek L.; Parry M. A.; Shewry P. R.; Halford N. G. Asparagine in plants. Ann. Appl. Biol. 2007, 150, 1–26. [Google Scholar]
- Curtis T. Y.; Muttucumaru N.; Shewry P. R.; Parry M. A.; Powers S. J.; Elmore J. S.; Mottram D. S.; Hook S.; Halford N. G. Evidence for genetic and environmental effects on free amino acid levels in wheat grain: implications for acrylamide formation during processing. J. Agric. Food Chem. 2009, 57, 1013–1021. [DOI] [PubMed] [Google Scholar]
- Granvogl M.; Wieser H.; Koehler P.; Von Tucher S.; Schieberle P. Influence of sulfur fertilization on the amounts of free amino acids in wheat. Correlation with baking properties as well as with 3-aminopropionamide and acrylamide generation during baking. J. Agric. Food Chem. 2007, 55, 4271–4277. [DOI] [PubMed] [Google Scholar]
- De Wilde T.; De Meulenaer B.; Mestdagh F.; Govaert Y.; Vandeburie S.; Ooghe W.; Fraselle S.; Demeulemeester K.; Van Peteghem C.; Calus A.; Degroodt J. M.; Verhe R. Influence of fertilization on acrylamide formation during frying of potatoes harvested in 2003. J. Agric. Food Chem. 2006, 54, 404–408. [DOI] [PubMed] [Google Scholar]
- Kumar D.; Singh B. P.; Kumar P. An overview of the factors affecting sugar content of potatoes. Ann. Appl. Biol. 2004, 145, 247–256. [Google Scholar]
- Eppendorfer W. H.; Bille S. W. Free and total amino acid composition of edible parts of beans, kale, spinach, cauliflower and potatoes as influenced by nitrogen fertilisation and phosphorus and potassium deficiency. J. Sci. Food Agric. 1996, 71, 449–458. [Google Scholar]
- Elmore J. S.; Mottram D. S.; Muttucumaru N.; Dodson A. T.; Parry M. A.; Halford N. G. Changes in free amino acids and sugars in potatoes due to sulfate fertilization, and the effect on acrylamide formation. J. Agric. Food Chem. 2007, 55, 5363–5366. [DOI] [PubMed] [Google Scholar]
- Sowokinos J.Stress-Induced Alterations in Carbohydrate Metabolism; CAB International: Wallingford, UK, 1990. [Google Scholar]
- Nursten H. E.The Maillard Reaction; Royal Society of Chemistry: Cambridge, UK, 2005. [Google Scholar]
- Mottram D. S.The Maillard reaction: source of flavor in thermally processed foods. In Flavors and Fragrances: Chemistry, Bioprocessing and Sustainability; Berger R. G., Ed.; Springer-Verlag: Berlin, Germany, 2007; pp 269–284. [Google Scholar]
- Mottram D. S.; Wedzicha B. L.; Dodson A. T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [DOI] [PubMed] [Google Scholar]
- Stadler R. H.; Blank I.; Varga N.; Robert F.; Hau J.; Guy P. A.; Robert M.-C.; Riediker S. Acrylamide from Maillard reaction products. Nature 2002, 419, 449–450. [DOI] [PubMed] [Google Scholar]
- Zyzak D. V.; Sanders R. A.; Stojanovic M.; Tallmadge D. H.; Eberhart B. L.; Ewald D. K.; Gruber D. C.; Morsch T. R.; Strothers M. A.; Rizzi G. P.; Villagran M. D. Acrylamide formation mechanism in heated foods. J. Agric. Food Chem. 2003, 51, 4782–4787. [DOI] [PubMed] [Google Scholar]
- Granvogl M.; Jezussek M.; Koehler P.; Schieberle P. Quantitation of 3-aminopropionamide in potatoes – a minor but potent precursor in acrylamide formation. J. Agric. Food Chem. 2004, 52, 4751–4757. [DOI] [PubMed] [Google Scholar]
- Claus A.; Weisz G. M.; Schieber A.; Carle R. Pyrolytic acrylamide formation from purified wheat gluten and gluten-supplemented wheat bread rolls. Mol. Nutr. Food Res. 2006, 50, 87–93. [DOI] [PubMed] [Google Scholar]
- Granvogl M.; Schieberle P. Thermally generated 3-aminopropionamide as a transient intermediate in the formation of acrylamide. J. Agric. Food Chem. 2006, 54, 5933–5938. [DOI] [PubMed] [Google Scholar]
- Amrein T. M.; Bachmann S.; Noti A.; Biedermann M.; Barbosa M. F.; Biedermann-Brem S.; Grob K.; Keiser A.; Realini P.; Escher F.; Amadò R. Potential of acrylamide formation, sugars, and free asparagine in potatoes: a comparison of cultivars and farming systems. J. Agric. Food Chem. 2003, 51, 5556–5560. [DOI] [PubMed] [Google Scholar]
- Becalski A.; Lau B. P.-Y.; Lewis D.; Seaman S. W.; Hayward S.; Sahagian M.; Ramesh M.; Leclerc Y. Acrylamide in French fries: influence of free amino acids and sugars. J. Agric. Food Chem. 2004, 52, 3801–3806. [DOI] [PubMed] [Google Scholar]
- Shepherd L. V. T.; Bradshaw J. E.; Dale M. F. B.; McNicol J. W.; Pont S. D. A.; Mottram D. S.; Davies H. V. Variation in acrylamide producing potential in potato: segregation of the trait in a breeding population. Food Chem. 2010, 123, 568–573. [Google Scholar]
- Elmore J. S.; Dodson A. T.; Briddon A.; Halford N. G.; Mottram D. S.. The effects of storage on the formation of aroma and acrylamide in heated potato. In Controlling Maillard Pathways to Generate Flavors; Mottram D. S., Taylor A. J., Eds.;American Chemical Society: Washington, DC, 2010; pp 95–109. [Google Scholar]
- Halford N. G.; Muttucumaru N.; Powers S.; Gillatt P. N.; Hartley S.; Elmore J. S.; Mottram D. S. Concentrations of free amino acids and sugars in nine potato varieties: effects of storage and relationship with acrylamide formation. J. Agric. Food Chem. 2012, 60, 12044–12055. [DOI] [PubMed] [Google Scholar]
- Parker J. K.; Balagiannis D. P.; Higley J.; Smith G.; Wedzicha B. L.; Mottram D. S. Kinetic model for the formation of acrylamide during the finish-frying of commercial French fries. J. Agric. Food Chem. 2012, 60, 9321–9331. [DOI] [PubMed] [Google Scholar]
- Results on acrylamide levels in food from monitoring years 2007–2009 and exposure assessment. EFSA J. 2011, 9, 2133. [Google Scholar]
- Powers S. J.; Mottram D. S.; Halford N. G.. Acrylamide concentrations in potato crisps in Europe from 2002 to 2011. Food Addit. Contam.: Part A 2013, DOI: 10.1080/19440049.2013.805439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muttucumaru N.; Elmore J. S.; Curtis T.; Mottram D. S.; Parry M. A. J.; Halford N. G. Reducing acrylamide precursors in raw materials derived from wheat and potato. J. Agric. Food Chem. 2008, 56, 6167–6172. [DOI] [PubMed] [Google Scholar]
- Halford N. G.; Curtis T. Y.; Muttucumaru N.; Postles J.; Elmore J. S.; Mottram D. S. The acrylamide problem: a plant and agronomic science issue. J. Exp. Bot. 2012, 63, 2841–2851. [DOI] [PubMed] [Google Scholar]
- Roach J. A. G.; Andrzejewski D.; Gay M. L.; Nortrup D.; Musser S. M. Rugged LC-MS/MS survey analysis for acrylamide in foods. J. Agric. Food Chem. 2003, 51, 7547–7554. [DOI] [PubMed] [Google Scholar]
- Riley N. G.; Zhao F. J.; McGrath S. P. Leaching losses of sulphur from different forms of sulphur fertilizers: a field lysimeter study. Soil Use Manage. 2002, 18, 120–126. [Google Scholar]
- Zhao F. J.; Hawkesford M. J.; McGrath S. P. Sulphur assimilation and effects on yield and quality of wheat. J. Cereal Sci. 1999, 30, 1–17. [Google Scholar]
- Postles J.; Powers S.; Elmore J. S.; Mottram D. S.; Halford N. G. Effects of variety and nutrient availability on the acrylamide forming potential of rye grain. J. Cereal Sci. 2013, 57, 463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis T. Y.; Powers S. J.; Balagiannis D.; Elmore J. S.; Mottram D. S.; Parry M. A. J.; Raksegi M.; Bedő Z.; Shewry P. R.; Halford N. G. Free amino acids and sugars in rye grain: implications for acrylamide formation. J. Agric. Food Chem. 2010, 58, 1959–1969. [DOI] [PubMed] [Google Scholar]
- Bråthen E.; Kita A.; Knutsen S. H.; Wicklund T. Addition of glycine reduces the content of acrylamide in cereal and potato products. J. Agric. Food Chem. 2005, 53, 3259–3264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


