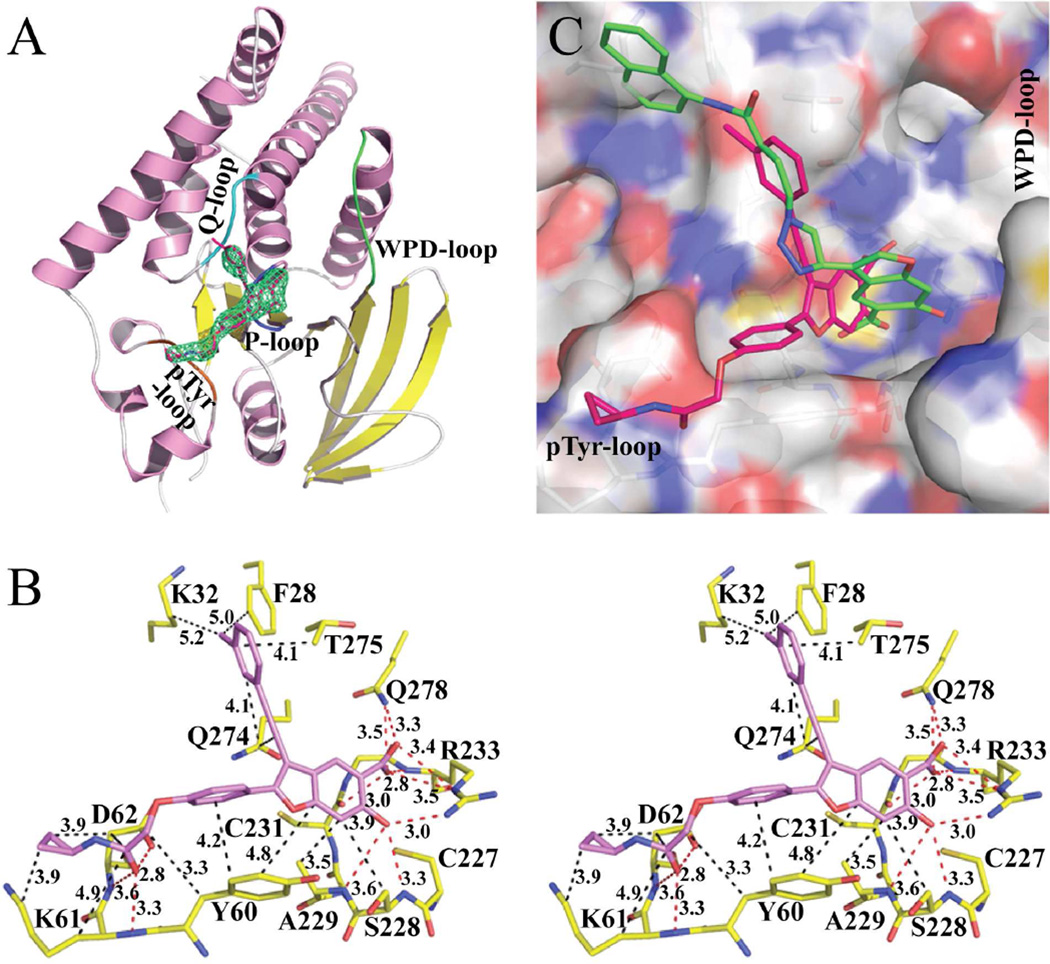
Figure 4.
Crystal structure of LYP in complex with compound 8b. (A). Overall structure of LYP catalytic domain in complex with 8b. α helices and β strands are colored in pink and yellow, respectively. The P-loop is shown in blue, the WPD-loop in green, the Q-loop in cyan, and the pTyr-loop in orange. Compound 8b is shown in stick model with unbiased Fo-Fc map contoured at 3.0σ calculated before the ligand and water molecules were added to the model. (B). Detailed interactions between compound 8b and LYP. Polar interactions or H-bonds are shown by red dashed lines; hydrophobic interactions are shown in black dashed lines. Residues involved in polar or hydrophobic interactions are shown with a cutoff distance of 3.6 and 5.2 Å, respectively. (C). Binding mode comparison between compound 8b and compound 2. The superposition of LYP•8b and LYP•2 was calculated with active site residues without the ligands. LYP active site was shown by transparent surface representation, and the key residues are depicted in stick model. Atomic colors were as follows: oxygen – red, carbon – white, sulfur – orange, and nitrogen – blue. Carbon atoms of 8b were colored red, and compound 2’s carbon atoms were colored green. For 2, the phenyl ring on the 2-position of the benzofuran core was invisible in the crystal structure.