Figure1.
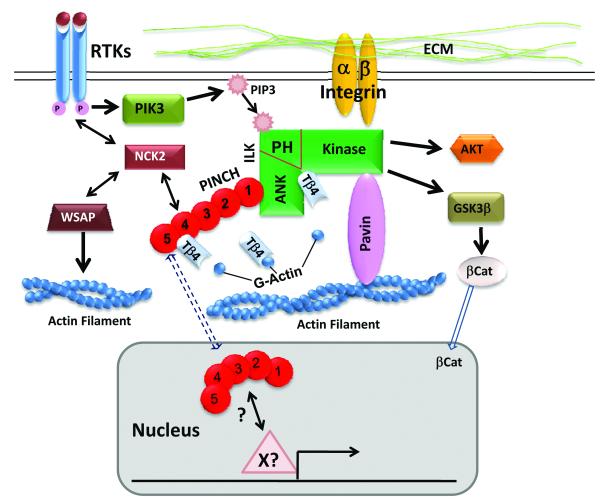
Cell adhesions play important roles in cell growth, differentiation, migration and survival. Engagement of integrin with extracellular matrix (ECM) leads to the recruitment and formation of a cytoplasmic focal adhesion complex composed of integrin-linked kinase (ILK), PINCH and Parvin, which links ECM-integrins to the actin cytoskeleton. PINCH, a five LIM domain (1-5) adaptor protein, interacts with the ankyrin domain (ANK) of ILK via its LIM1 domain and interacts with NCK2 via its LIM4 domain, thus Links integrin pathway to other intracellular pathways, especially growth factor-receptor tyrosine kinase (RTK) pathways. ILK kinase is activated in a PI3 kinase (PI3K) dependent manner, which in turn activated downstream PKB/AKT required for cell growth and survival. ILK also phosphorylates and inhibits GSK3b, leading to the stabilization and translocation of b-catenin to the nucleus and activation of gene expression. Thymosin β4 (Tβ4) formed a functional complex with PINCH and ILK via direct interactions with PINCH and ILK, resulting in activation of Akt and promoting myocardial cell migration, survival and repair. In the neural crest cells, PINCH is predominantly nuclear, suggesting a direct role of PINCH in regulating gene expression in complex with transcription factor(s) yet to be identified (X?) .