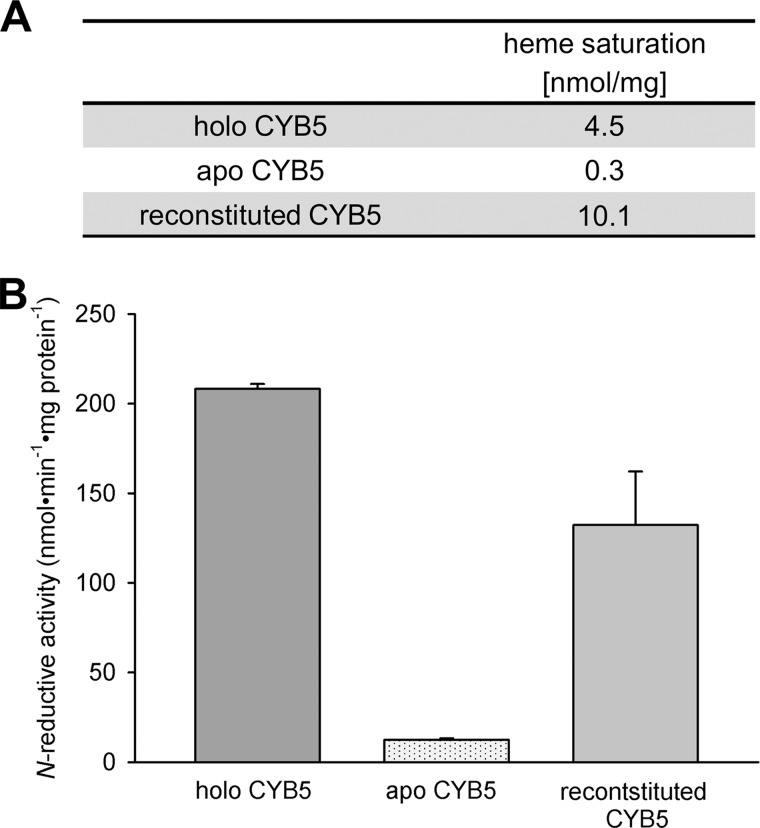
FIGURE 6.
Involvement of cytochrome b5 heme in N-reduction. Recombinant CYB5 (holo-CYB5) was gained as described under “Experimental Procedures.” Apo-CYB5 was prepared from holo-CYB5 by heme cleavage, and afterward the same batch was reconstituted by heme incorporation. The human recombinant N-reductive enzyme system was then reconstituted with mARC1, CYB5R, and either form of CYB5. N-Reductive activities were measured with benzamidoxime as a model substrate. A, heme saturations of holo-CYB5, apo-CYB5, and reconstituted CYB5 were determined to confirm the success of heme cleavage and heme incorporation. B, N-reductive activities of holo-CYB5, apo-CYB5, and reconstituted CYB5 were determined as described under “Experimental Procedures.” The incubation mix contained 75 pmol of mARC, 7.5 pmol of CYB5R, and 75 pmol of CYB5 in the case of holo and reconstituted CYB5 or the same amount of protein as holo-CYB5 in the case of apo-CYB5. Incubations were performed in duplicates. Results are presented as means ± S.D.