FIGURE 9.
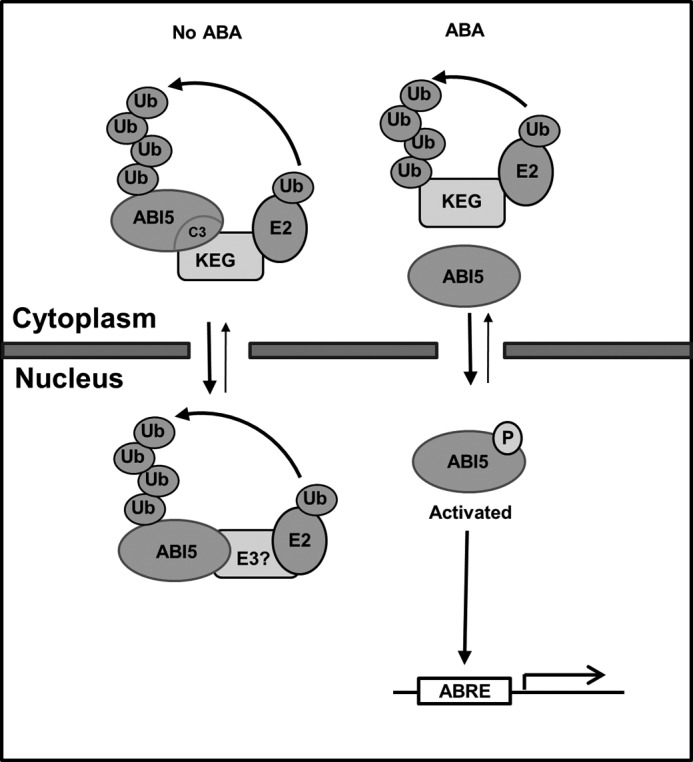
Model for regulating ABI5 protein abundance in the absence and presence of ABA. In the absence of ABA, KEG interacts with the C3 domain of ABI5, ubiquitinates and targets ABI5 for degradation by the proteasome in the cytoplasm. Another E3 may regulate the abundance of nuclear ABI5 utilizing amino acids 410–442. In response to ABA, ABI5 accumulates due to increase transcription and impediment of KEG E3 activity by ABA-mediated KEG self-ubiquitination and degradation. ABI5 is activated by phosphorylation, which leads to the expression of ABA-responsive genes. When the activity of ABI5 is no longer required or needs to be reduced, the transcription factor is degraded by other E3s such as DWA1/2 containing Cullin-based RING E3 ligase.
