Abstract
The treatment of osteochondral articular defects has been challenging physicians for many years. The better understanding of interactions of articular cartilage and subchondral bone in recent years led to increased attention to restoration of the entire osteochondral unit. In comparison to chondral lesions the regeneration of osteochondral defects is much more complex and a far greater surgical and therapeutic challenge. The damaged tissue does not only include the superficial cartilage layer but also the subchondral bone. For deep, osteochondral damage, as it occurs for example with osteochondrosis dissecans, the full thickness of the defect needs to be replaced to restore the joint surface 1. Eligible therapeutic procedures have to consider these two different tissues with their different intrinsic healing potential 2. In the last decades, several surgical treatment options have emerged and have already been clinically established 3-6.
Autologous or allogeneic osteochondral transplants consist of articular cartilage and subchondral bone and allow the replacement of the entire osteochondral unit. The defects are filled with cylindrical osteochondral grafts that aim to provide a congruent hyaline cartilage covered surface 3,7,8. Disadvantages are the limited amount of available grafts, donor site morbidity (for autologous transplants) and the incongruence of the surface; thereby the application of this method is especially limited for large defects.
New approaches in the field of tissue engineering opened up promising possibilities for regenerative osteochondral therapy. The implantation of autologous chondrocytes marked the first cell based biological approach for the treatment of full-thickness cartilage lesions and is now worldwide established with good clinical results even 10 to 20 years after implantation 9,10. However, to date, this technique is not suitable for the treatment of all types of lesions such as deep defects involving the subchondral bone 11.
The sandwich-technique combines bone grafting with current approaches in Tissue Engineering 5,6. This combination seems to be able to overcome the limitations seen in osteochondral grafts alone. After autologous bone grafting to the subchondral defect area, a membrane seeded with autologous chondrocytes is sutured above and facilitates to match the topology of the graft with the injured site. Of course, the previous bone reconstruction needs additional surgical time and often even an additional surgery. Moreover, to date, long-term data is missing 12.
Tissue Engineering without additional bone grafting aims to restore the complex structure and properties of native articular cartilage by chondrogenic and osteogenic potential of the transplanted cells. However, again, it is usually only the cartilage tissue that is more or less regenerated. Additional osteochondral damage needs a specific further treatment. In order to achieve a regeneration of the multilayered structure of osteochondral defects, three-dimensional tissue engineered products seeded with autologous/allogeneic cells might provide a good regeneration capacity 11.
Beside autologous chondrocytes, mesenchymal stem cells (MSC) seem to be an attractive alternative for the development of a full-thickness cartilage tissue. In numerous preclinical in vitro and in vivo studies, mesenchymal stem cells have displayed excellent tissue regeneration potential 13,14. The important advantage of mesenchymal stem cells especially for the treatment of osteochondral defects is that they have the capacity to differentiate in osteocytes as well as chondrocytes. Therefore, they potentially allow a multilayered regeneration of the defect.
In recent years, several scaffolds with osteochondral regenerative potential have therefore been developed and evaluated with promising preliminary results 1,15-18. Furthermore, fibrin glue as a cell carrier became one of the preferred techniques in experimental cartilage repair and has already successfully been used in several animal studies 19-21 and even first human trials 22.
The following protocol will demonstrate an experimental technique for isolating mesenchymal stem cells from a rabbit's bone marrow, for subsequent proliferation in cell culture and for preparing a standardized in vitro-model for fibrin-cell-clots. Finally, a technique for the implantation of pre-established fibrin-cell-clots into artificial osteochondral defects of the rabbit's knee joint will be described.
Keywords: Biomedical Engineering, Issue 75, Medicine, Anatomy, Physiology, Cellular Biology, Molecular Biology, Stem Cell Biology, Tissue Engineering, Surgery, Mesenchymal stem cells, fibrin clot, cartilage, osteochondral defect, rabbit, experimental, subchondral bone, knee injury, bone grafting, regenerative therapy, chondrocytes, cell culture, isolation, transplantation, animal model
Protocol
A. Preparation of a Donor Rabbit for the Isolation of Mesenchymal Stem Cells (Surgery Room)
Cells are isolated from male New Zealand White (NZW) rabbits at age of 4 months and approximately 3 kg body weight.
Induce anesthesia by propofol (10 mg/kg body weight i.v.) and sacrifice with sodium pentobarbital (100 mg/kg body weight i.v.).
Shave fur from hind limbs, back and belly with an electric clipper and vacuum the fur.
Disinfect the shaved area thoroughly with 70% ethanol.
Use blunt forceps, sharp scissors (or scalpel) and bone cutters for tissue and ligaments.
Make an incision along the cranial surface of the leg and calf.
Reflect skin and subcutaneous tissue by either sharp or blunt dissection.
Separate muscles and ligaments from tibia and femur. Keep cuts as close to the bone as possible to make a clean dissection. Do not separate femur from tibia at this time.
Cut through the hip joint to separate the head of the femur from the acetabulum.
Elevate the tibial-femoral complex.
Use the scalpel blade to scrape off any remaining soft tissue from the bones or rub the bones with sterile cloth tissues. At this point, femur and tibia are still connected.
Remove patella by cutting, then cut knee joint ligaments to separate bones finally.
Spray separated bones with 70% ethanol, let air dry and place each bone into a 50 ml centrifuge tube with cell culture medium (DMEM + 1% penicillin/streptomycin (Pen/Strep)) to keep them moist.
Now switch under a sterile cell culture laminar flow hood.
B. Flushing of Rabbit MSC from Bones and Expansion (Cell Culture Hood)
Collect bones from tubes and place them into 150 mm dishes using sterile forceps.
Remove both bone ends with a sterile saw and move pieces to new 150 mm dishes.
Fill a 10 ml syringe with medium (DMEM), attach an 18 gauge needle and insert into the opening of the bone marrow.
Then, rinse marrow cavity with medium to flush the bone marrow into the dish. Afterwards, rinse from the other end, if possible. If necessary, saw off more from the ends. If bone should break, just rinse the inside of the bone.
Aspirate cell medium suspension into the syringe and rinse the bone marrow repeatedly until suspension is free floating through the bone marrow cavity and no further bone marrow-clots appear.
Once bone marrow has been collected from all bones, disrupt the marrow clumps by passing through an 18 gauge needle: fill syringe with needle attached and force out into medium.
Afterwards, filter the suspension through a cell filter into a 50 ml tube. In order to prevent cell loss, wash culture dish 2x with 10 ml medium and filter as well.
Centrifuge suspension at 500 x g for 5 min at RT.
Remove supernatant and resuspend cell pellet in 10 ml medium (DMEM + 1% Pen/Strep).
Separate blood cells from Peripheral Blood Mononuclear Cells (PBMC) and mesenchymal stem cells (MSC) using a Biocoll Separating Solution.
Fill 5 ml of Biocoll Separating Solution into a 15 ml tube and carefully add 5 ml of cell-suspension on top and centrifuge at 800 x g for 20 min at RT (without brake).
Possible results see Figure 1: Being denser than Biocoll, red blood cells sediment to the bottom while PBMC and mesenchymal stem cells remain at the interface.
Carefully remove interface into a 15 ml tube and wash with 5 ml PBS.
Centrifuge as described in step 22, resuspend in 5 ml PBS and repeat 2-3x.
Then, centrifuge again at 350 x g for 10 min at RT (with brake).
Resuspend in 10 ml medium and count cells in a hemocytometer.
Plate cells at an initial seeding density of approximately 5 x 106 in 150 mm dishes.
After 2-3 days, remove non-adherent cells. You might have to rinse with PBS first in order to remove cell debris. Add fresh complete medium (DMEM + 10% Fetal Calf Serum (FCS) + 1% Pen/Strep) afterwards.
Feed cells every 3-4 days (Figure 2).
After 5-10 days, passage the cells for the first time.
C. Preparation of Fibrin Clots in vitro
At the day of implantation, release adherent cells from flasks/dishes by a 3 min-exposure to 0.25% trypsin-EDTA. Stop trypsinization by adding complete medium.
Distribute cells in a 50 ml falcon tube and wash them twice with PBS.
Determine cell viability and numbers by trypan blue staining.
Add 50,000 cells/microcentrifuge tube and collect pellets by centrifugation at 500 x g for 5 min at RT. Prepare a mastermix for at least one more clot as needed.
Resuspend the cell pellet in 17 μl PBS and mix 25 μl of the fibrinogen component of TISSUCOL-Kit with this 17 μl MSC suspension.
Take a sterile plate with pre-drilled holes (3x3.6 mm) in accordance to the drill holes in vivo (Figure 3).
First, inoculate 4 μl thrombin solution (500 IU/ml) into one hole, followed by immediate addition of 42 μl fibrinogen-cell-suspension and again 4 μl thrombin solution on top. Do not mix the suspension to avoid clotting in the pipette tip. First, the 50 μl volume of the pipetted fibrinogen-cell-suspension will protrude the rim of the pre-drilled holes without melting due to the surface tension. However, after complete clotting (after 60 min) the clot is contracting and fits into the pre-drilled hole.
Remove the clot carefully using a blunt forceps and place into a microcentrifuge tube with PBS. Prepare 2 clots/animal.
Take the clots to the surgery room.
D. Implantation of Allogeneic Mesenchymal Stem Cells in Fibrin Clots
Induce anesthesia to rabbit (NZW, male, 3.5-4.0 kg body weight, 5-6 months old) by i.v. injection of propofol (10 mg/kg body weight).
Shave the knee to be operated on with an electric clipper and vacuum the fur. All the procedures named before are performed in a surgery preparation room to avoid contamination of the sterile environment of the operating room.
After intubation, maintain anesthesia with 1.5 mg/kg/min propofol and 0.05 mg/kg/min fentanyl intravenously. Monitor anesthesia by using capnography, pulse oximetry and pulse rate.
Disinfect the shaved knee thoroughly and cover the rest of rabbit with a sterile dressing.
Palpate the patella and perform a skin incision medial to the patella.
Open the knee joint by a medial parapatellar arthrotomy under sterile conditions. Try to avoid cutting any small superficial blood vessels.
Displace the patella laterally (Figure 4).
After inspection of the knee joint for any concomitant cartilage lesions or joint anomalies, create two osteochondral defects (3 mm deep, figure eight-shaped) in the trochlear groove with a sterile air operating power drill (3.6 mm in diameter) with a stop-device (Figure 5) .
Clean the defects and rinse them with sterile saline.
Prior to implantation, fill 20 μl of fibrin glue into the defects and distribute them evenly onto the bottom of the defect.
Then implant the clots press-fitted into the figure eight-shaped defect.
After clotting, relocate the patella within the trochlear groove and bend and stretch the knee a few times.
Displace the patella laterally once again and check if fibrinogen-cell-clots are still in place.
Replace the patella again and finish the operation with wound closure in layers with single button sutures (4-0 Vicryl) and a continuous cutaneous suture (4-0 Monocryl) (both with absorbable suture material).
Finally, seal the wound with a spray dressing permeable to water vapor.
For post-operative care, the wound is checked daily for 7 days. The rabbits receive for post-operative analgesia Carprofen 4 mg/kg s.c. every 24 hr (for 4 days) and Buprenorphin 0.03 mg/kg s.c. every 12 hr (for 2.5 days). A stabilization of the knee (e.g. dressing) is not necessary.
Representative Results
The described surgical technique permits a successful isolation and implantation of allogeneic mesenchymal stem cells into an artificial osteochondral defect. The experimental setup resulted in a successful integration of the implant into the surrounding cartilage.
The defect was filled by repair tissue with similar biomechanical properties and similar durability compared to the surrounding cartilage. The fibrin-cell-clot was prepared in vitro on a sterile plate with pre-drilled holes, which had the same size as the osteochondral defect (Figure 3). As a result, there were no clefts between the implanted fibrin clot and the surrounding cartilage, which would be a risk factor for premature degeneration or delamination (Figure 6). A basal healing of the repair tissue was ensured because the subchondral bone was penetrated, and thus worked against a shearing. Another important aspect was the stiffness of the repair tissue, which should match the healthy surrounding cartilage tissue to avoid an increased load on it and a possible premature degeneration. In our preliminary experiments (data not shown), we showed that after 12 weeks a sufficient rigidity could already be achieved. Moreover, an intact and homogeneous surface of the transplant was found, which reduced shear stress and possible implant damage (Figure 6).
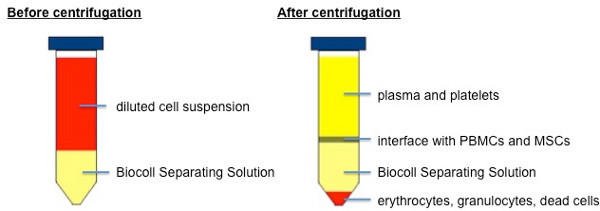 Figure 1. Separation of blood cells and plasma from PBMCs and MSCs using Biocoll Separating Solution.
Figure 1. Separation of blood cells and plasma from PBMCs and MSCs using Biocoll Separating Solution.
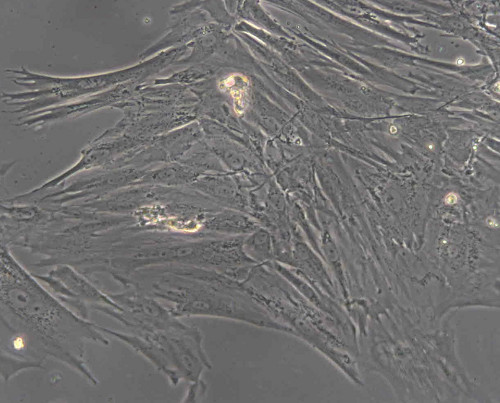 Figure 2. Monolayer of mesenchymal stem cells (New Zealand White rabbit) after 5 days in culture.
Figure 2. Monolayer of mesenchymal stem cells (New Zealand White rabbit) after 5 days in culture.
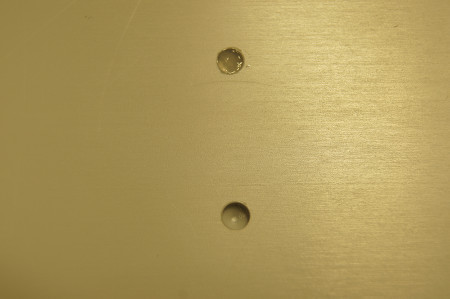 Figure 3. Sterile plate with pre-drilled holes (3x3.6 mm) the top one being filled with a fibrin-cell-clot.
Figure 3. Sterile plate with pre-drilled holes (3x3.6 mm) the top one being filled with a fibrin-cell-clot.
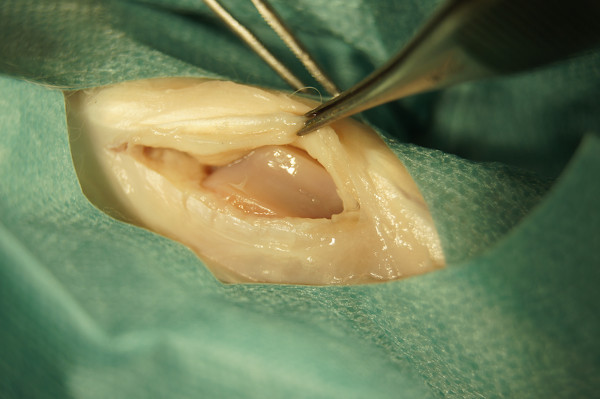 Figure 4. Opened knee joint after medial parapatellar arthrotomy.
Figure 4. Opened knee joint after medial parapatellar arthrotomy.
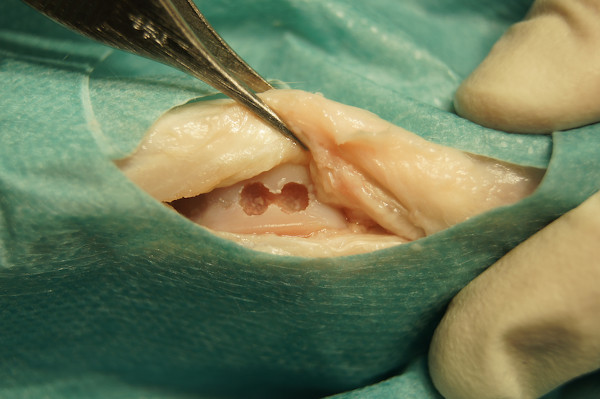 Figure 5. Osteochondral defects (3 mm deep, 3 mm in diameter, figure eight-shaped) in the trochlear groove.
Figure 5. Osteochondral defects (3 mm deep, 3 mm in diameter, figure eight-shaped) in the trochlear groove.
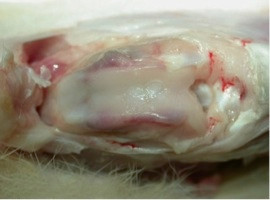 Figure 6. Opened knee joint 12 weeks after implantation of two fibrin-cell-clots into two drilled osteochondral defects.
Figure 6. Opened knee joint 12 weeks after implantation of two fibrin-cell-clots into two drilled osteochondral defects.
Discussion
In recent years, the possibility of treating complex articular osteochondral defects - such as those resulting from osteochondritis dissecans, osteonecrosis and trauma - with Tissue Engineering approaches became more and more attractive. In the previously mentioned pathologic entities, tissue damage extends to the subchondral bone and involves two tissues characterized by different intrinsic healing capacities 1. There is an increasing interest in the role of subchondral bone for the pathogenic processes of osteochondral articular damage 11,23. The functional conditions of articular cartilage and its supporting bone are tightly connected. Injuries of either tissue adversely affect the mechanical environment as well as the homeostatic balance of the entire joint 24. Alterations in the osteochondral unit through mechanical disruption of joint motion, loose body formation, mechanical wear in the involved compartment and attrition of opposing surfaces may lead to an earlier onset and development of osteoarthritis 1,11. Therefore, tissue engineering approaches for the regeneration of osteochondral defects should be accompanied by an adequate restoration of the underlying subchondral bone in order to enhance the effective union with surrounding host tissues 2. Mesenchymal stem cells seem to be an ideal cell source to provide these specific requirements of osteochondral repair. The protocol highlights the promotion of bone and cartilage tissue restoration by potentially inducing the selective differentiation of the transplanted mesenchymal stem cells in osteogenic and chondrogenic lineages.
In comparison to chondrocytes, mesenchymal stem cells have several major advantages: they can be easily isolated from bone marrow, synovialis and fat tissue without any greater donor side morbidity. Mesenchymal stem cells do not differentiate during in vitro expansion and therefore can be culture-expanded in large numbers to treat large articular cartilage defects. Moreover, they seem to be immunosuppressive and - in response to appropriate stimuli - can differentiate into chondrocytes and osteocytes 25,26. Another remarkable advantage of mesenchymal stem cells displays their hypoimmunity, which means that allogeneic mesenchymal stem cells can be used without any sign of rejection reaction 27. Therefore, a cell-pool from one or two donor animals can be sufficient for all experiments. This reduces operation time and additional harm to the animals.
Several experiments showed promising results using fibrin glue for osteochondral repair 19,20. Usually, the inoculation of the fibrinogen-cell-suspension with thrombin solution has been described as a procedure done in situ, directly into the artificial osteochondral lesions. After a short period of time of pre-clotting (after a few minutes) the operation is usually finished by relocating the patella and wound closure.
In several pilot tests, we found out that for sufficient clotting of the fibrin-cell-suspension it takes more than 60 min. In situ - during the operation - it is hardly possible to wait more than 60 min for entire clotting. Moreover, by use of a sterile plate with pre-drilled holes simulating the osteochondral lesions, it was possible to show that the amount of fibrin glue, which was used to obviously fill the defect completely, was not enough due to shrinking of the hardening clot. This requires a higher volume of fibrin glue in advance in order to achieve a congruent and complete filling of the defect. Performing the preparation of the clot in vitro it is possible to easily adjust the construct shape to the appropriate size of the drilled defect and therefore, fill the osteochondral defect totally and congruently.
Additionally, an in vitro preparation of the cell clots prevents a leakage of the adhesive but (after only a few minutes) not fully hardened fibrin-cell-suspension. Therefore, it can be guaranteed that the initially intended volume will stay in the defect and will begin with cartilage integration and remodeling.
The described technique permits a standardized method for experimental stem cell research in the field of osteochondral repair. The protocol provides a reproducible way to isolate mesenchymal stem cells in order to re-implant them later in osteochondral cartilage defects of the rabbit's knee joint. Autologous chondrocytes have already been implanted into osteochondral defects in a fibrin-cell-model 19. Using an in vitro-model of clot preparation as well as mesenchymal stem cells instead of chondrocytes appears to be a more advantageous and promising new approach for remodeling and repair of osteochondral lesions.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This project was funded by the German Research Association (grant HE 4578/3-1) and partially by the FP7 EU-Project “GAMBA” NMP3-SL-2010-245993.
References
- Kon E, et al. Novel nano-composite multilayered biomaterial for osteochondral regeneration: a pilot clinical trial. The American Journal of Sports Medicine. 2011;39:1180–1190. doi: 10.1177/0363546510392711. [DOI] [PubMed] [Google Scholar]
- Kon E, et al. Orderly osteochondral regeneration in a sheep model using a novel nano-composite multilayered biomaterial. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society. 2010;28:116–124. doi: 10.1002/jor.20958. [DOI] [PubMed] [Google Scholar]
- Hangody L, et al. Autologous osteochondral grafting--technique and long-term results. Injury. 2008;39:32–39. doi: 10.1016/j.injury.2008.01.041. [DOI] [PubMed] [Google Scholar]
- Marcacci M, et al. Arthroscopic autologous osteochondral grafting for cartilage defects of the knee: prospective study results at a minimum 7-year follow-up. The American Journal of Sports Medicine. 2007;35:2014–2021. doi: 10.1177/0363546507305455. [DOI] [PubMed] [Google Scholar]
- Ochs BG, et al. Remodeling of articular cartilage and subchondral bone after bone grafting and matrix-associated autologous chondrocyte implantation for osteochondritis dissecans of the knee. The American Journal of Sports Medicine. 2011;39:764–773. doi: 10.1177/0363546510388896. [DOI] [PubMed] [Google Scholar]
- Aurich M, et al. Autologous chondrocyte transplantation by the sandwich technique. A salvage procedure for osteochondritis dissecans of the knee. Unfallchirurg. 2007;110:176–179. doi: 10.1007/s00113-006-1174-6. [DOI] [PubMed] [Google Scholar]
- Williams RJ, 3rd, Ranawat AS, Potter HG, Carter T, Warren RF. Fresh stored allografts for the treatment of osteochondral defects of the knee. The Journal of Bone and Joint Surgery. American Volume. 2007;89:718–726. doi: 10.2106/JBJS.F.00625. [DOI] [PubMed] [Google Scholar]
- Szerb I, Hangody L, Duska Z, Kaposi NP. Mosaicplasty: long-term follow-up. Bull. Hosp. Jt. Dis. 2005;63:54–62. [PubMed] [Google Scholar]
- Brittberg M, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am. J. Sports Med. 2010;38:1117–1124. doi: 10.1177/0363546509357915. [DOI] [PubMed] [Google Scholar]
- Gomoll AH, et al. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg. Sports Traumatol. Arthrosc. 2010;18:434–447. doi: 10.1007/s00167-010-1072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhagen J, et al. Treatment of osteochondritis dissecans of the femoral condyle with autologous bone grafts and matrix-supported autologous chondrocytes. Int. Orthop. 2010;34:819–825. doi: 10.1007/s00264-009-0841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, et al. Repair of large articular cartilage defects with implants of autologous mesenchymal stem cells seeded into beta-tricalcium phosphate in a sheep model. Tissue Eng. 2004;10:1818–1829. doi: 10.1089/ten.2004.10.1818. [DOI] [PubMed] [Google Scholar]
- Centeno CJ, et al. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11:343–353. [PubMed] [Google Scholar]
- Niederauer GG, et al. Evaluation of multiphase implants for repair of focal osteochondral defects in goats. Biomaterials. 2000;21:2561–2574. doi: 10.1016/s0142-9612(00)00124-1. [DOI] [PubMed] [Google Scholar]
- Nagura I, et al. Repair of osteochondral defects with a new porous synthetic polymer scaffold. J. Bone. Joint Surg. Br. 2007;89:258–264. doi: 10.1302/0301-620X.89B2.17754. [DOI] [PubMed] [Google Scholar]
- Schlichting K, et al. Influence of scaffold stiffness on subchondral bone and subsequent cartilage regeneration in an ovine model of osteochondral defect healing. The American Journal of Sports Medicine. 2008;36:2379–2391. doi: 10.1177/0363546508322899. [DOI] [PubMed] [Google Scholar]
- Schagemann JC, et al. Cell-laden and cell-free biopolymer hydrogel for the treatment of osteochondral defects in a sheep model. Tissue Engineering. Part A. 2009;15:75–82. doi: 10.1089/ten.tea.2008.0087. [DOI] [PubMed] [Google Scholar]
- Vogt S, et al. The influence of the stable expression of BMP2 in fibrin clots on the remodelling and repair of osteochondral defects. Biomaterials. 2009;30:2385–2392. doi: 10.1016/j.biomaterials.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Schillinger U, et al. A fibrin glue composition as carrier for nucleic acid vectors. Pharm. Res. 2008;25:2946–2962. doi: 10.1007/s11095-008-9719-8. [DOI] [PubMed] [Google Scholar]
- Ahmed TA, Giulivi A, Griffith M, Hincke M. Fibrin glues in combination with mesenchymal stem cells to develop a tissue-engineered cartilage substitute. Tissue Engineering. Part A. 2011;17:323–335. doi: 10.1089/ten.TEA.2009.0773. [DOI] [PubMed] [Google Scholar]
- Haleem AM, et al. The Clinical Use of Human Culture-Expanded Autologous Bone Marrow Mesenchymal Stem Cells Transplanted on Platelet-Rich Fibrin Glue in the Treatment of Articular Cartilage Defects: A Pilot Study and Preliminary Results. Cartilage. 2010;1:253–261. doi: 10.1177/1947603510366027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape D, Filardo G, Kon E, van Dijk CN, Madry H. Disease-specific clinical problems associated with the subchondral bone. Knee Surg Sports Traumatol. Arthrosc. 2010;18:448–462. doi: 10.1007/s00167-010-1052-1. [DOI] [PubMed] [Google Scholar]
- Shirazi R, Shirazi-Adl A. Computational biomechanics of articular cartilage of human knee joint: effect of osteochondral defects. Journal of Biomechanics. 2009;42:2458–2465. doi: 10.1016/j.jbiomech.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Jorgensen C, Gordeladze J, Noel D. Tissue Engineering through autologous mesenchymal stem cells. Curr. Opin. Biotechnol. 2004;15:406–410. doi: 10.1016/j.copbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Chen FH, Tuan RS. Mesenchymal stem cells in arthritic diseases. Arthritis Res. Ther. 2008;10:223. doi: 10.1186/ar2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]


