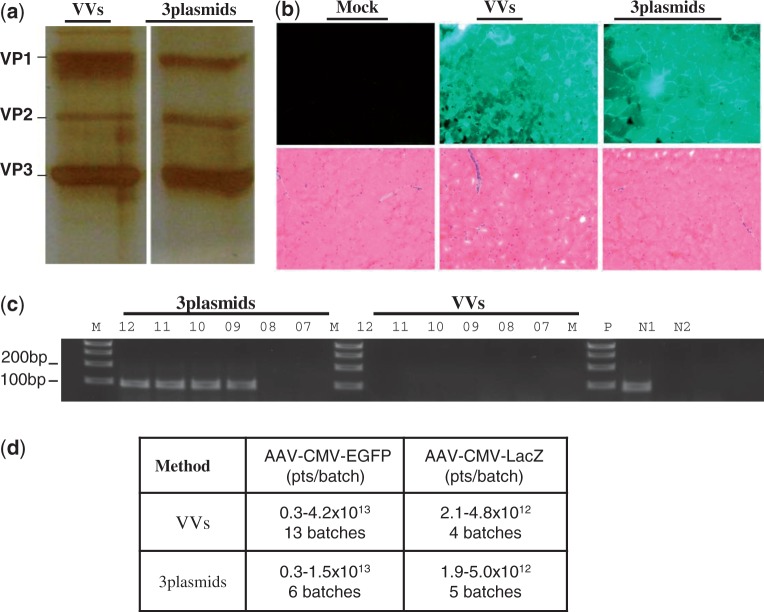
Figure 4.
Scalable production of AAV vectors using vaccinia carrier and Ad/AAV hybrid in suspension cells. The suspension HeLa S3 cells were infected by Ad-AAV-CMV-EGFP and VVs (vv-Rep78, 52, vv-VP1 and vv-VP2). The cells were harvested at 36 h after infection and the rAAV vectors were purified by two rounds of CsCl gradient ultracentrifugation. (a) Silver staining of rAAV vector produced by vaccinia carriers or triple transfection methods (‘3plasmids’). Each lane includes 1 × 1010 purified AAV particles (pts) resolved on 10% SDS-PAGE. (b) AAV-CMV-GFP transduction of mouse muscle. C57BL/6 mice were injected with 5 × 1010 purified viral pts produced by the above procedure. The tissue was harvested at 9 weeks after injection. Sections (10 µm in thickness) were subjected to HE staining (lower) or direct GFP signal visualization under fluorescent microscope. (c) Detection of rcAAV after sequential amplification in HEK293 cells. Hirt DNA was extracted and used as the template for PCR (35 cycles) with primers specific for AAV coding region and separated on agarose gel. Vectors produced by triple plasmid transfection method (‘3plasmids’) and vaccinia carriers (‘VVs’) were analyzed. ‘P’, PCR positive control using plasmid template; N1, infection with Ad only; N2, infection with 1 × 1012 rAAV vectors only. The numbers on top of the gel lanes indicate the amount of vectors in logs being used for initial infection of HEK293 cells. (d) Summary of rAAV vector yield by the vaccinia helper (4VVs and 2VVs) as compared with triple plasmid transfection. The vector yield ranges and number of batches attempted are shown.