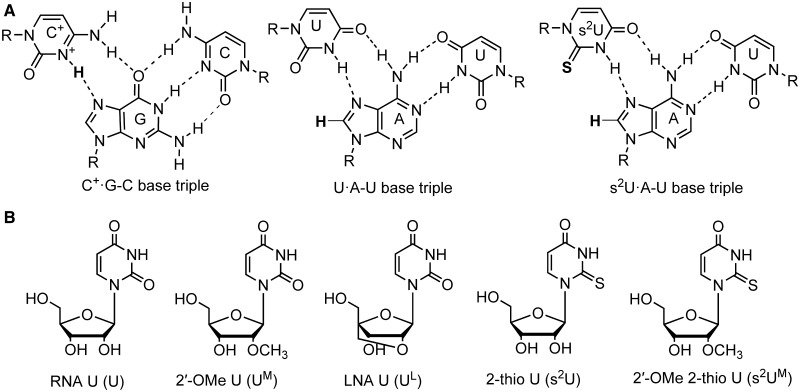
Figure 1.
(A) Base triples studied in this article. The N3 atom of C base needs to be protonated (shown in bold) to form C+·G–C base triple. The s2U·A–U base triple is more stable than unmodified U·A–U base triple because of enhanced van der Waals interaction between the thio group in 2-thio U and H8 hydrogen (shown in bold) in A and other molecular interactions. (B) Chemical structures of nucleosides: RNA U (U), 2′-OMe U (UM), LNA U (UL), 2-thio U (s2U) and 2′-OMe 2-thio U (s2UM).