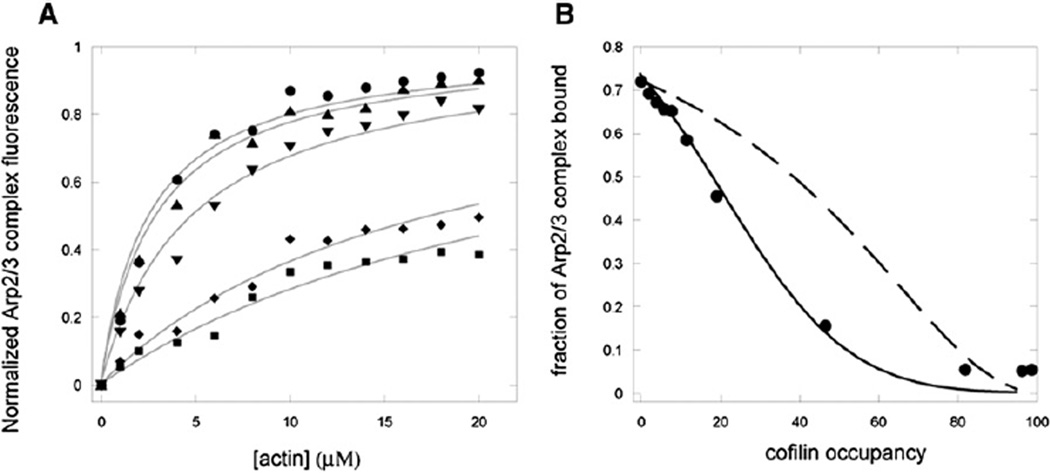
Figure 3. Cofilin Reduces the Affinity of Arp2/3 Complex for ADP-Actin Filaments.
(A) Dependence of equilibrium fluorescence of 600 nM pyrenyl-Arp2/3 complex on the concentration of ADP-actin filaments and on saturation of the filaments with cofilin: circles, no cofilin; triangles, 10% saturation; inverted triangles, 20% saturation; rhombuses, 30% saturation; squares, 45% saturation. Conditions: 600 nM Arp2/3 complex, 10 mM imidazole (pH 7), 2 mM Tris-HCl, 1 mM MgCl2, 1 mM EGTA, 0.2 mM ATP, 0.5 mM DTT, 1 mM NaN3, 50 mM KCl incubated over night at 4°C with a range of concentrations of ADP-actin filaments and cofilin. Fluorescence was measured at room temperature.
(B) Dependence of the normalized equilibrium fluorescence of pyrenyl-Arp2/3 complex with 5 µM ADP-actin filaments on the fractional saturation of the filaments with cofilin. Filled circles: Experimental observations with 600 nM pyrenyl Arp2/3 complex, 5 µM ADP-actin filaments, and a range of cofilin concentrations incubated in KEMI-F buffer for 24 hr at 4°C. The fraction of actin-bound Arp2/3 complex was calculated from the increase in fluorescence relative to the initial value. The dashed line is a theoretical calculation of fraction bound Arp2/3 complex assuming direct competition between Arp2/3 complex and cofilin for actin filaments. Here cofilin competes solely by limiting the number of 3-subunit binding sites for Arp2/3 complex by a factor of (1 – c)1.94 as described in Equation 5 and Figure S1. Arp2/3 complex has a constant affinity (KDR = 0.67 µM) for filaments whereas cofilin associates with increasing affinity (Kd = 8.2 µM to 0.11 µM), as calculated from the fractional occupancy and free cofilin concentration in Equation 7. The solid line is another theoretical calculation of fraction bound Arp2/3 complex assuming both competition for binding sites (Equation 5) and a cofilin-induced structural propagation in the filament that increases the affinity of cofilin (Kd = 8.2 µM to 0.11 µM) and decreases the affinity of Arp2/3 complex (KDR = 0.67 µM to 23 µM, calculated from Equation 6) for actin filaments as filaments change from zero to full cofilin occupancy.