Fig. 6.
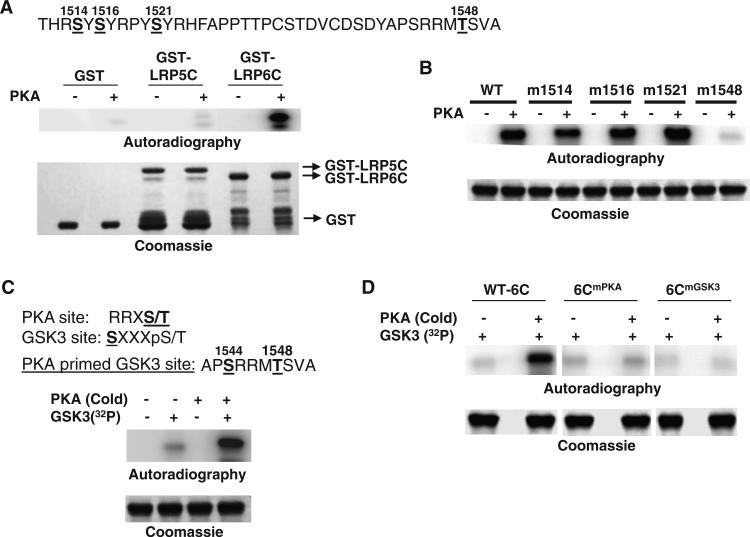
Phosphorylation of LRP6 by PKA enhances the binding of LRP6 to Gαs. (A) PKA directly phosphorylates the intracellular domain of LRP6, as determined by in vitro kinase assay. The consensus PKA phosphorylation sites in the intracellular domain of LRP6 are shown at the top. GST, GST-LRP5C, and GST-LRP6C proteins were pulled down by glutathione beads. The bead-bound proteins were incubated with the recombinant catalytic subunit of PKA and [γ-32P]ATP, and the protein-associated radiolabel was visualized by PhosphorImager analysis (Autoradiography). The GST proteins were analyzed by SDS-PAGE and visualized by Coomassie Brilliant Blue staining. Data are representative of three experiments. (B) Mutation of Thr1548 of LRP6 abolishes the phosphorylation of LRP6C by PKA. WT or various point-mutated GST-6C proteins were pulled down by glutathione beads. The bead-bound proteins were incubated with the catalytic subunit of PKA and [γ-32P]ATP, and the protein-associated radiolabel was analyzed by SDS-PAGE and visualized by PhosphorImager analysis (Autoradiography). The GST proteins were visualized by Coomassie Brilliant Blue staining. Data are representative of three experiments. (C) Phosphorylation of LRP6C by PKA primes its phosphorylation by GSK-3. The consensus PKA and GSK-3 phosphorylation sites are shown at the top. GST-6C proteins were pulled down by glutathione beads. The bead-bound proteins were incubated with the catalytic subunit of PKA and cold ATP, washed, and then incubated with recombinant GSK-3 and [γ-32P]ATP. The protein-associated radiolabel was analyzed by SDS-PAGE and visualized by PhosphorImager analysis (Autoradiography). The GST proteins were visualized by Coomassie brilliant blue staining. Data are representative of three experiments. (D) Mutation of the PKA or GSK-3 sites of LRP6 abolishes the sequential phosphorylation of LRP6C by PKA and GSK-3. WT (WT-6C) or the point-mutated GST-6C proteins, 6CmPKA (Thr1548→Ala) and 6CmGSK3 (Ser1544→Ala), were pulled down by glutathione beads. The bead-bound proteins were then treated and analyzed as described for (A). The GST proteins were visualized by Coomassie Brilliant Blue staining. Data are representative of three experiments. Abbreviations for the amino acids are as follows: A, Ala; C, Cys; D, Asp; F, Phe; H, His; M, Met; P, Pro; R, Arg; S, Ser; T, Thr; V, Val; and Y, Tyr.