Abstract
The purpose of this study was to test the bond strength and analyze the morphology of the dentin-adhesive interface of two etch and rinse and two self-etch adhesive systems with two kinds of artificial saliva (with and without 450 mg/L mucin) contamination under different conditions of decontaminating the interface. Bonded specimens were sectioned perpendicularly to the bonded surface in 1-mm thick slabs. These 1-mm thick slabs were remounted in acrylic blocks and sectioned in sticks perpendicular to the bonding interfaces with a 1-mm2 area. Nine specimens from each condition were tested after 24 h on a testing machine (Instron) at a speed of 0.5 mm/min for a total of 360 specimens. Mean and standard deviations of bond strength (MPa) were calculated. ANOVA showed significant differences as well as Fisher’s PLSD intervals (p < 0.05). The following values are the results for different groups: Control group 34–60 MPa, saliva without mucin 0–52 MPa, and saliva with mucin 0–57 MPa. Failure sites were mixed and adhesive failure was common for the low bond strength results. P&BNT with ideal conditions and following the manufacturer’s instructions (control) had the highest bond strengths and the dentin-adhesive interface exhibited an ideal morphology of etch-and-rinse system. SEM gave complementary visual evidence of the effect in the dentin/adhesive interface structure with some contaminated conditions compared with their respective control groups. This in vitro artificial saliva model with and without mucin showed that an organic component of saliva could increase or decrease the bond strength depending on the specific bonding agent and decontamination procedure.
Keywords: Bond strength, Saliva, Mucin, SEM, Self etching systems
Introduction
With the new self-etching adhesive systems, the components of the smear layer may form a portion of the bonding substrate [1]. The primers in these systems are acidic enough to demineralize most or all of the smear layer and the top layer of the underlying dentin surface. As they etch, they also infiltrate the exposed collagen with hydrophilic monomers, which then co-polymerize with the subsequently placed adhesive resin. The concept of an acidic primer is attractive, because in theory, this system allows for simultaneous infiltration of the exposed collagen matrix as it decalcifies the inorganic component of the dentin. This technique should minimize exposed collagen at the dentin/adhesive interface [2].
Clinically, one of the factors that can affect adhesion and retention of restorations is contamination of the restorative field [3]. Some of the common contaminants in clinical practice are saliva, blood, astringents, water, hand piece lubricant, zinc oxide-eugenol cement, and non-eugenol cement [4]. Some authors have reported a decrease in the bond strength in the presence of plasma and saliva [5, 6]. Other authors have reported that plasma decreases the bond strength by 33 and 70% on enamel and dentin, respectively. The protein content in the blood is a critical aspect in the decrease of the bond strength [6–8].
Human saliva is a complex mixture of fluids from three major salivary glands, minor salivary glands as well as gingival crevicular fluids. The amount of secretion is 1–1.5 L/day. The range of pH of unstimulated saliva is 5.8–7.1, with an average value of 6.38. As the secretion rate increases, the pH value also increases [8, 10, 11]. Some of the organic substances found in saliva are amylase and lysozyme [9]. The protein content of saliva is 1.34 ± 1.10 g/L. The concentration of each protein is as follows: albumin 25 mg/L; gamma-globulin 50 mg/L; and mucoprotein 450 mg/L. The concentration of amylase is 0.42 ± 0.06 µg/L, and that of lysozyme is 140 µg/L. Mucins are the principal organic constituents of mucus. Mucins coat the mucosal surfaces to form a viscoelastic pellicle [10–13]. Mucins have important functions in the oral cavity such as remineralization, lubrication, bolus formation, taste, antifungal and antiviral agents, antibacterial agents, aggregation and clearance of microorganisms [11, 13, 14].
Microscopic examination of acid-etched dentin contaminated with saliva indicated it did not prevent hybrid layer formation; however, it did reduce the adaptation of the restorative material to bonded surfaces [14]. Several studies reported significant decreases in tooth surface adhesion in the presence of saliva contamination [6, 15]. Other studies have reported tolerance to saliva contamination. It has been suggested that moisture on the dentin surface could prevent salivary proteins from penetrating the dentin tubules and occluding them [17]. It has also been suggested that the water content of the saliva might increase the hydration of the dentin surface, thus producing favorable conditions for the performance of acetone-based dental adhesives [18]. In addition, air used to thin the adhesive and to help speed the solvent evaporation may assist with the infiltration of the adhesive through the saliva and into the dentinal tubules [19].
When acetone-based primers come into contact with moist dentin, the relative vapor pressure of acetone increases because the attractive force between acetone molecules is higher than the attractive force between the water molecules. Theoretically, acetone and water then evaporate from the substrate leaving the resin behind [20]. Previous studies have suggested that water-based primers do not provide comparable results. For example, water-based primers have been shown to prevent HEMA molecules from saturating the collagen mesh [21]. This failure was related to the observation that water-based primers do not evaporate as easily or as completely as acetone-based adhesives. Moreover, the presence of water inside the collagen mesh might reduce the polymerization of HEMA which results in a weaker hybrid layer [21, 22].
When composite resin was bonded directly to saliva-contaminated enamel etched with phosphoric acid, a reduction of more than 50% in mean bond strength was found [16]. In bonding to dentin contaminated with saliva, a reduction in bond strength between 40 and 100% has been reported. Scanning electron microscopy analysis of these saliva-contaminated specimens did not show evidence of the formation of a hybrid layer and resin tags were not found in the tubules [16].
Some self-etching adhesive systems seem to be more compatible with the oral environment for several reasons that include low sensitivity to moisture and saliva contamination [5, 23, 24]. Furthermore, other authors reported that water-solvent adhesives are less sensitive to variations in surface moisture [25]. These reports led us to speculate that the reason for the low sensitivity of the self-etching systems to saliva is the water solvent of the primer.
It has been reported that the protein content of the saliva is responsible for the decrease in adhesive bond strength when contamination with saliva has occurred [17, 26, 27]. The purpose of this study was to test the bond strength and analyze the morphology of the dentin/adhesive interface of several adhesive systems with two kinds of artificial saliva contamination under different conditions of decontaminating the interface. The null hypothesis was that various decontamination procedures for dentin surfaces contaminated with artificial saliva (with or without mucin) resulted in (1) no significant differences in bond strength and (2) no alterations in interface morphology of various adhesive systems.
Materials and methods
Products, batch numbers, expiration dates, and manufacturers of the materials used in this study are listed in Table 1. Ingredients, manufacturers, and quantity of the ingredients in the artificial saliva are shown in Table 2. A protein extracted from bovine submaxillary glands [mucin (450 mg/L)] was added to the artificial saliva to determine if mucin would affect the bond strength of the different bonding agents used in the study. The artificial saliva that contained 450 mg/L of mucin in order to simulate the natural contain of mucin in human saliva previously characterized by other researchers [10, 11, 13].
Table 1.
Products, manufacturers, lot numbers, and expiration dates of the products used in the study
| Product | Manufacturer | Batch number | Expiration date |
|---|---|---|---|
| Adper Single Bond Plus (SBP) | 3M ESPE | 5BX | 06-2008 |
| Filtek Supreme | 5FG | 04-2008 | |
| Primer & Bond NT (P&BNT) | Dentsply Caulk | 041101 | 03-2008 |
| TPH Spectrum | 041117 | 03-2008 | |
| Clearfil SE Bond-Primer (SEB) | Kuraray America | 00482A | 04-2008 |
| Clearfil SE Bond–Bond (SEB) | 00669A | 04-2008 | |
| Clearfil APX | 000854B | 07-2008 | |
| Clearfil S3 (S3) | 00001A | 08-2009 | |
| 34% tooth conditioner | Dentsply Caulk | 030514 | 09-2008 |
| Mucin from bovine submaxillary glands, Type I–S | SIGMA | 044k7048 | 10-2008 |
Table 2.
Chemical composition of the artificial saliva
| Ingredients | Manufacturer | Quantity |
|---|---|---|
| Distilled water | Prev Dent | 90 L |
| Methyl paraben, NF/FCC | Ashland | 200.0 g |
| Sodium carboxymethylcellulose (NaCMC) | Aqualon | 1000.0 g |
| Potassium chloride | EM Science | 62.5 g |
| Magnesium chloride hexahydrate | Fisher | 5.875 g |
| Calcium chloride dihydrate | 16.625 g | |
| Potassium phosphate (dibasic, anhydrous) | 80.375 g | |
| Potassium phosphate (monobasic) | J.T. Baker | 32.625 g |
| Sorbitol solution, 70%, USP/FCC | ICI Amer | 4275.0 g |
| FD&C Red Dye #40 (2% sterilized) | N–B & Wolfe | 25 mL |
One hundred and forty human third molars were stored in 0.25% sodium azide/saline solution for no more than 3 months. Before the specimens’ preparation we randomly assigned five teeth to the control groups and the three contamination conditions using artificial saliva with or without mucin. The teeth were ground parallel to the occlusal surface with 60-grit SiC paper (Carbimet Paper Disc, Buehler, Lake Forest, IL, USA) immediately before bonding on a polishing machine (Ecomet 6, Buehler, Lake Forest, IL, USA) to expose superficial dentin and then finished with 600-grit SiC paper (Carbimet Paper Disc, Buehler, Lake Forest, IL, USA). The specimens were randomly bonded following the manufacturers’ instructions for the control groups and according to one of the three contamination conditions using artificial saliva with or without mucin for each of the four adhesives with their respective composites (Prime&Bond NT/TPH Spectrum, Denstply/Caulk, Milford, DE, USA; Adper Single Bond Plus/Filtek Supreme, 3M ESPE, St. Paul, MN, USA; Clearfil SE Bond/Clearfil APX, Clearfil S3/Clearfil APX, Kuraray America, Japan).
Conditions
Control group following manufacturers’ instructions
10 µL of saliva was dispensed on the dentin surface followed by the application of the different adhesive systems to the wet surface (S)
10 µL of saliva was dispensed on the dentin surface and was air dried followed by the application of the different adhesive systems (SA)
10 µL of saliva was dispensed on the dentin surface and was rinsed with water and air dried followed by the application of the different adhesive systems (SW).
Photopolymerization was accomplished with a halogen light-curing unit (Optilux 501, SDS/Kerr Demetron, Orange, CA, USA). The light output was verified with a curing radiometer (Demetron Research Corp., Danbury, CT, USA) to be at a level >770 mW/cm2 throughout the study.
The bonded specimens were stored in distilled water for 24 h at 37°C in an incubator. After storage, each specimen was mounted in acrylic blocks using epoxy resin (Cole-Parmer Instruments Co, Vernon Hills, IL, USA) then sectioned in half perpendicularly to the bonded surface half of each tooth was randomly chosen and stored under room temperature conditions for further SEM. The other half was sectioned perpendicularly to the bonded surface in 1-mm thick slabs. These 1-mm thick slabs were again remounted in epoxy blocks and sectioned to form sticks perpendicular to the bonding interfaces, resulting in 1-mm2 bonding areas.
Nine specimens out of five teeth restored following the contamination conditions and two control groups for each of the four adhesive systems were randomly selected as follows: two sticks from four teeth and one stick from the fifth tooth for a total of 252 specimens. These nine randomly selected specimens were used for microtensile bond strength testing. Each specimen was glued with cyanoacrylate adhesive (Zap-It, DVA, Corona, CA, USA). The cyanoacrylate was applied far from the portion of the specimens that was tested to failure and to the opposing arms of a specialized microtensile jig. Each specimen was de-bonded in tension using a testing machine (Instron Model 4465, Instron Corp., Canton, MA, USA) at a crosshead speed of 0.5 mm/min. Forces (kg) at failure were recorded. The failure mode percentages of the debonded specimens were measured using a microscope (Optispec Microscope, Enterprise, Norcross, GA, USA) at 100× magnification and then averaged. The bond strength (MPa) was calculated for each specimen.
Means of bond strengths were compared using three-way analysis of variance. Fisher PLSD intervals at the 0.05 level of significance were used to determine significant differences between saliva with and without mucin, among adhesives systems, and among bonding conditions (S, SA, SW).
SEM analysis
Half of each tooth used for bond strength testing was randomly chosen and stored under room temperature conditions. The specimens were sectioned perpendicular to the adhesive interface with a slow-speed diamond saw (Isomet, Buehler, Lake Forest, IL, USA) to produce two slices of 2–3 mm in thickness. One slice was acid-etched with 5 N HCl for 30 s followed by 5% NaOCl for 30 min and rinsed thoroughly with distilled water. The other slice was fractured perpendicular to the interface. Each slice was then dehydrated in successive concentrations of ethanol, i.e. 33, 67, and 85% ethanol for 30 min at each concentration and absolute ethanol for 60 min. The specimens were left overnight to dry and were then mounted on 12-mm aluminum stubs and sputter coated with approximately 20 nm of gold–palladium alloy. The specimens were viewed at 3 magnifications (1000×) and various tilt angles in a XL30 ESEM-FEG 515 field emission microscope at 10 kV (Philips Electron Optics Inc., Hillsboro, OR, USA). Analyses of the interface microstructure (hybrid layer, resin tag quality, and compactness of the different layers) were based on at least 20 images taken along the length of the dentin-adhesive interface.
Results
Means and standard deviations of the bond strengths are presented in Table 3. Analysis of variance (<0.0001) indicated significant differences. Fisher’s PLSD intervals for comparisons of means between mucin and no mucin, among adhesives, and among conditions were 1.6, 2.3, and 2.3 MPa, respectively.
Table 3.
Bond strengths of four adhesives when treated with and without mucin under different conditions
| Adhesive | Control | Without mucin | With mucin | ||||
|---|---|---|---|---|---|---|---|
| S | SA | SW | S | SA | SW | ||
| SEB | 52.9 (7.3)A | 51.8 (11.3)A | 19.7 (2.2)G | 11.1 (3.7) | 57.1 (8.8) | 19.9 (6.0)B,G | 21.5 (4.0)B,f |
| S3 | 34.3 (7.8) | 26.9 (5.7) | 12.9 (1.4) | 30.5 (4.5) | 20.0 (5.0) | 16.6 (3.9)e | 44.3 (9.8) |
| SBP | 48.3 (8.8) | 0.0 | 43.4 (6.4)C | 42.4 (6.4)C | 0.0 | 36.2 (10.6)D | 35.9 (7.1)D |
| P&BNT | 60.3 (13.3) | 0.0 | 0.0 | 35.3 (5.3) | 0.0 | 18.5 (5.6)e | 22.4 (7.0)f |
Means with standard deviations in parentheses (n = 9) of bond strength (MPa). Fisher’s PLSD intervals (p < 0.05) for comparisons of means between mucin and no mucin, among adhesives, and among conditions were 1.6, 2.3 and 2.3 MPa, respectively. Means with the same superscripted letters are statistically the same (upper case describes the rows and lower case describes the columns)
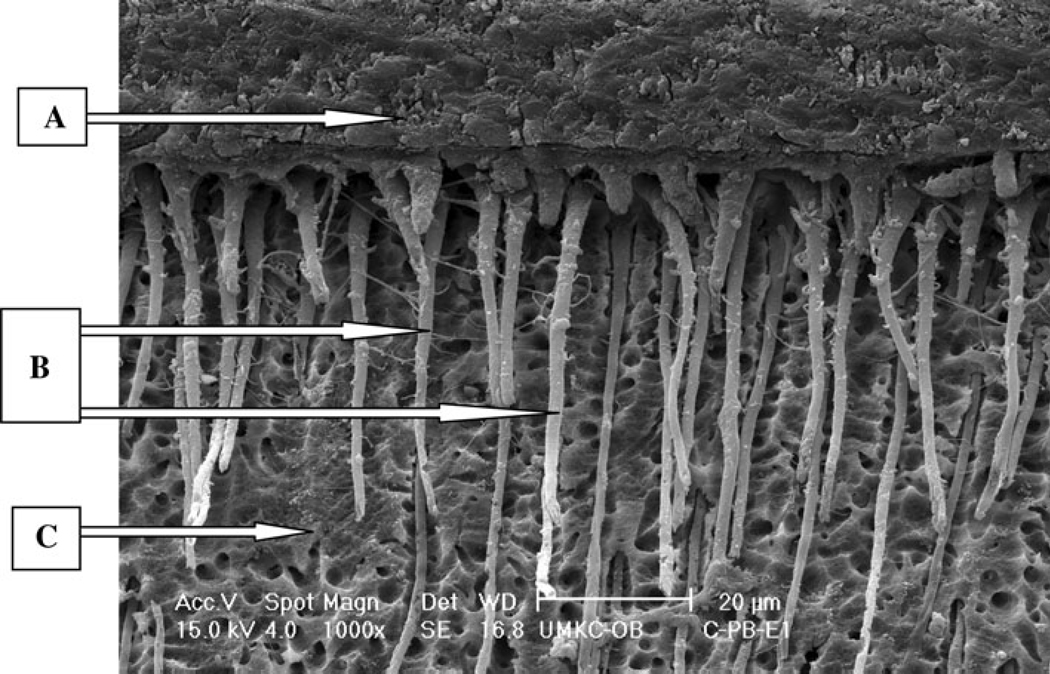
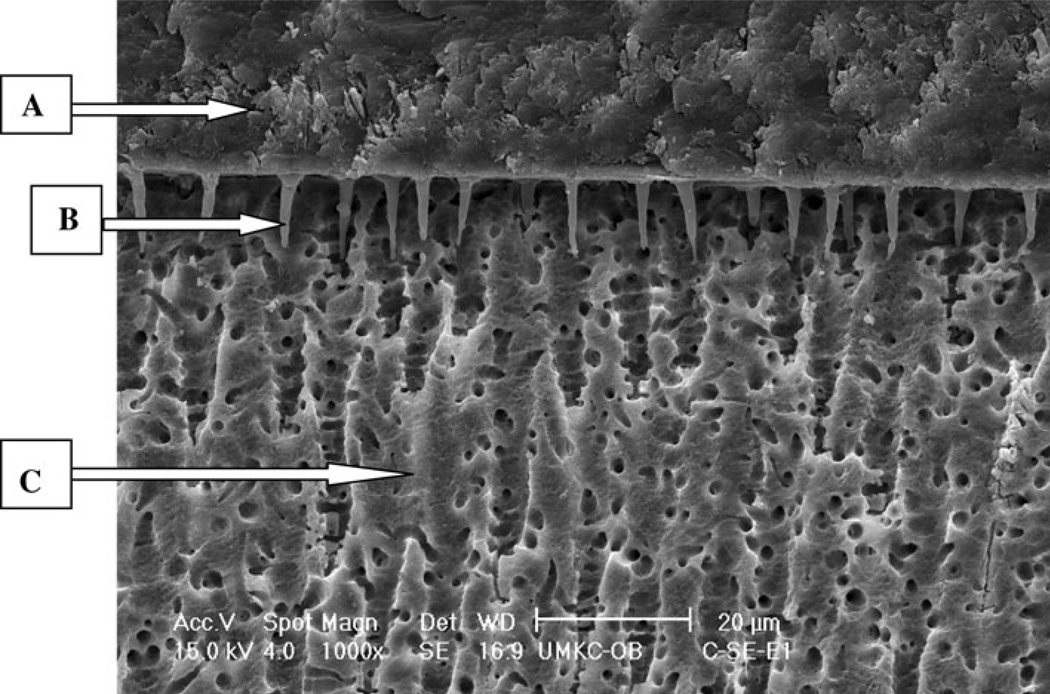
For the control groups, bond strength results were significantly different and ranked from highest to lowest as: P&BNT > SEB > SBP > S3. Scanning electron microscope (SEM) micrographs showed differences in the dentin/adhesive interface between the control groups of etch-and-rinse adhesive systems (P&BNT, SBP) and self-etch adhesive systems (SEB, S3). The etch-and-rinse adhesives (represented by P&BNT in Fig. 1) showed numerous, long resin tags all over the interface, whereas the two self-etch adhesive systems represented by SEB (Fig. 2) showed a totally different structure with the dentin/adhesive interface characterized by relatively few, short adhesive tags.
Fig. 1.
E-SEM (×1000) of a representative specimen with P&BNT under control condition with etch technique.
A Adhesive, B resin tags, C dentin
Fig. 2.
E-SEM (×1000) of a representative specimen with SEB under control condition with etch technique.
A Adhesive, B resin tag, C dentin
For condition S, the bond strengths with the etch-andrinse systems (P&BNT and SBP) showed zero values with and without mucin; these specimens remained bonded when placed into the fixtures, but failed at minimal loads (0.1–0.3 MPa). In comparison, the self-etching system S3 had bond strengths over 20 MPa with and without mucin. SEB bond strength results were statistically the same than the control group and SEB results with saliva without mucin were statistically higher than the control group.
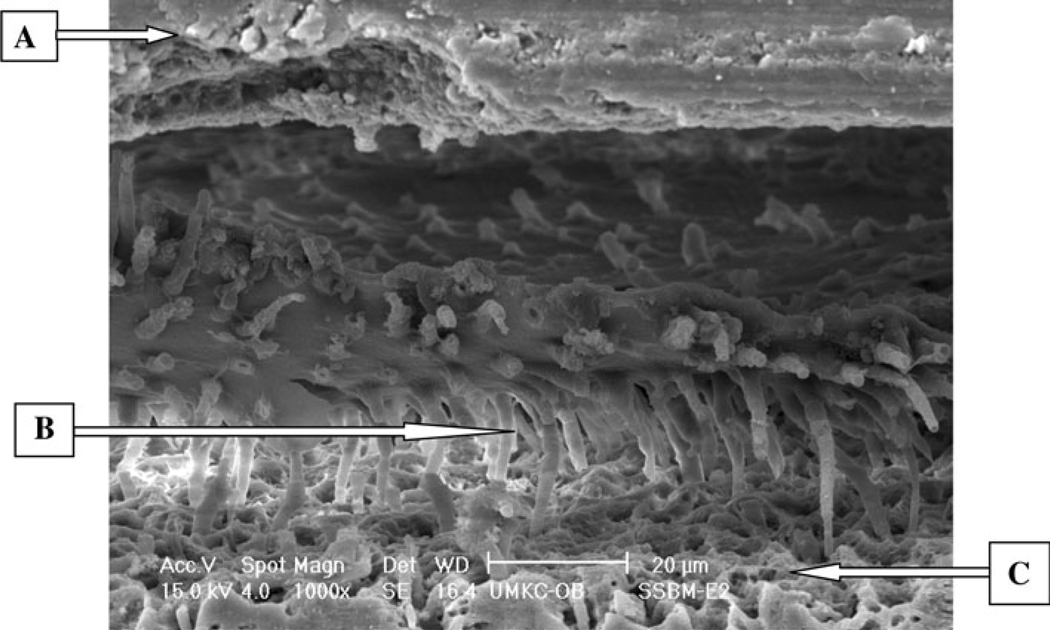
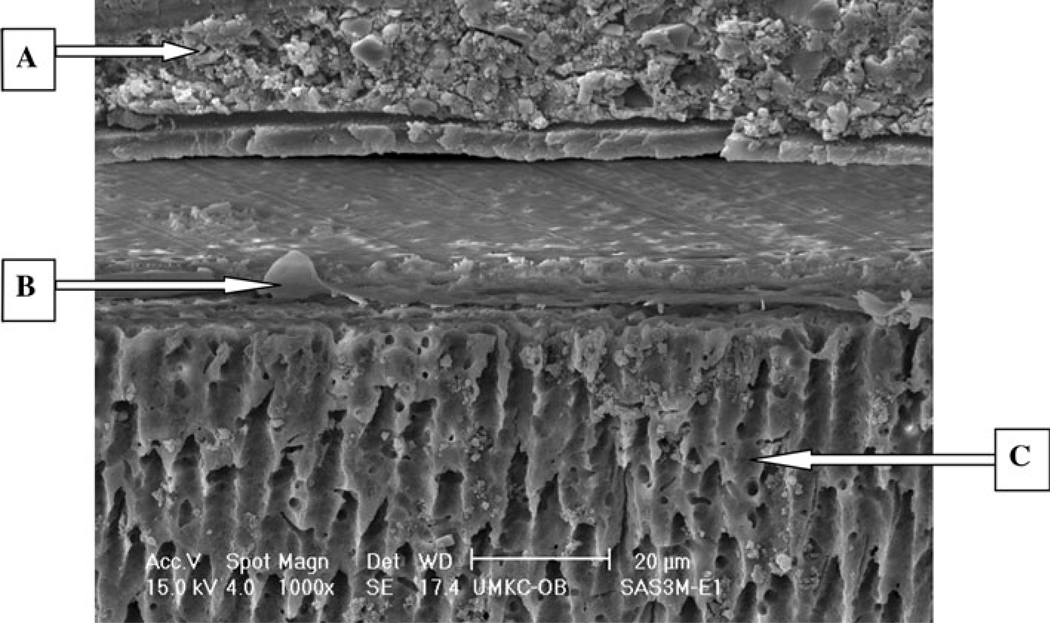
The SEM micrographs for the etch-and-rinse adhesives treated with mucin, represented by SBP (Fig. 3) showed numerous resin tags all over the interface, but there were cracks and separation at the interface between composite, adhesive and dentin substrates. The self-etching adhesives treated with mucin, as represented by SEB (Fig. 4), showed interfacial structures similar to their respective control groups.
Fig. 3.
E-SEM (×1000) of a representative specimen with SBP under S with mucin condition with etch technique.
A Adhesive, B resin tag, C dentin
Fig. 4.
E-SEM (×1000) of a representative specimen with SEB under S with mucin condition with etch technique.
A Adhesive, B resin tag, C dentin
For condition SA, SBP had the highest bond strengths for treatments with and without mucin. SBP showed slight, but significant decrease in bond strength as compared with its respective control group. Both self-etching systems (SEB and S3) with and without mucin showed statistically significant decreases in bond strength compared with their respective control groups. P&BNT with mucin showed a statistical decrease in bond strength compared with the control group and zero bond strength in the group without mucin.
SEM micrographs of SA treatment with P&BNT with and without mucin showed structures similar to the S condition, while SBP with and without mucin showed structures similar to the control group with numerous, long tags all over the interface (Fig. 5). SEB and S3 also had similar interface structures as seen in the S condition. S3 when treated without mucin showed longer tags compared with the other conditions including the control group, but when treated with mucin had an interface with cracks between the composite, adhesive and dentin structures (Fig. 6).
Fig. 5.
E-SEM (×1000) of a representative specimen with SBP under SA without mucin condition with etch technique.
A Adhesive, B resin tag, C dentin
Fig. 6.
E-SEM (×1000) of a representative specimen with S3 under SA with mucin condition with etch technique.
A Composite, B adhesive resin, C dentin
For condition SW, the adhesives SBP, P&BNT and S3 had bond strengths close to their respective control groups and exceeded 20 MPa when treated with and without mucin, except S3 that showed higher bond strengths with mucin than its control group. In comparison, for the SW condition SEB showed a decrease in bond strength when treated with and without mucin compared with the control group.
In general, the two self-etch systems (SEB, S3) when treated with and without mucin and imaged following the fracture technique for preparing specimens did not exhibit an identifiable hybrid layer. They showed a very compact adherent structure among composite, adhesive and dentin substrate. On the other hand, the two etch-and-rinse systems (P&BNT, SBP) when treated with and without mucin and imaged following the fracture technique, exhibited a hybrid layer and long resin tags. Other authors have described similar interface characteristics for both the etch-and-rinse and self-etch systems [28–30]. Failure mode analyses observed at 100× magnification are listed in Table 4. At the dissecting microscopic level (100×), the mode of failure observed was mainly at the adhesive interface.
Table 4.
At the dissecting scope level (×100), the mode of failure was observed mainly at the adhesive interface
| Adhesive | Control | With mucin | Without mucin | ||||
|---|---|---|---|---|---|---|---|
| S | SA | SW | S | SA | SW | ||
| SEB | 88A | 78A | 94A | 100A | 78A | 94A | 100A |
| 11C | 22C | 6C | 22C | 6C | |||
| S3 | 100A | 100A | 100A | 100A | 100A | 100A | 100A |
| SBP | 89A | 100A | 94A | 98A | 100A | 86A | 94A |
| 11C | 6C | 2C | 14C | 6C | |||
| P&BNT | 92A | 100A | 100A | 100A | 100A | 100A | 100A |
| 7C | |||||||
| 1D | |||||||
A adhesive, C cohesive in composite; D cohesive in dentin
Discussion
Based on the literature, there is no convincing evidence that the protein content of saliva is responsible for the decrease in bond strength of saliva-contaminated dentin/composite specimens; for that reason it is important to have a deeper understanding of the different components of the saliva, such as the organic substances and enzymes. In this study one protein component (mucin) of the saliva was evaluated by incorporating it (450 mg/L; the same concentration of mucin in human saliva) into artificial saliva in order to obtain a saliva model that could interact with the different adhesive systems to determine if this protein would affect the bond strength of the different adhesive systems used in the study.
SBP and P&BNT combine primer and solvent in a single bottle with the conditioner applied as a separate step. They have ethanol and acetone solvents, respectively. These solvents make them less sensitive to moist environments. In addition, the solvent is intended to facilitate the action of the adhesive in the presence of moist collagen. The wet bonding technique leads to moist collagen within the demineralized dentin matrix and the adhesive must penetrate this zone to accomplish adequate bonding with the subjacent mineralized dentin substrate [29]. SEM analysis of the control groups for these two etch and rinse bonding agents (SB and P&BNT) showed a formation of numerous, long resin tags and a thick hybrid layer (Fig. 1). This structure has been described as a typical adhesive/dentin microstructure for etch-and-rinse adhesive systems in other studies [28, 29].
The bond strengths with the etch-and-rinse adhesive systems contaminated with artificial saliva were similar to those previously reported in our group, exhibiting reduced bond strength [4]. Other studies have reported results that are in distinct contrast, i.e. etch-and-rinse systems are not sensitive to human saliva contamination when the wet bonding technique is used [17, 31, 32]. Those studies used human saliva as opposed to the artificial saliva used in the present study.
The results from this study showed that these two etch-and-rinse bonding agents were very sensitive to wet bonding when treated with and without mucin (S). The reasons for this detrimental effect on the bond strengths could be related to the components of the artificial saliva and the chemical interaction between the saliva and the components of the adhesive systems. In addition, the relative hydrophobic/hydrophilic nature of the adhesive impacts the bond formed at the interface with dentin as reported by other authors. The adhesive may experience phase separation in the presence of the wet demineralized dentin matrix [32, 33], with the adhesive/dentin interface forming a porous web of collagen with limited infiltration of the crosslinking hydrophobic adhesive component (BisGMA) [32]. This weak, porous structure created under wet bonding [22, 34] can be another explanation for the low bond strength results, along with the cracks and separation observed between composite, adhesive and dentin [28, 35–38]. Furthermore, it was evident in the SEM micrographs that the formation of the hybrid layer and numerous, long resin tags were not associated with improved bond strengths.
On the other hand, SEB with the same condition (S) showed the same or higher values than the control groups. SEB uses water as a solvent. The incorporation of water as a component of adhesive system would suggest that the product is more tolerant of water and thus potentially less sensitive to wet bonding. Furthermore, it is a two-bottle system, having the self-etching primer and the adhesive resin in separate containers. The artificial saliva with and without mucin mixed with the hydrophilic primer was air dried for 5 s before the application of the bonding agent. In other words, the hydrophobic bonding agent was not in contact with the surface when it was saturated with moisture.
S3 with the same condition (S) showed significant decrease in bond strength compared with the control groups. Although S3 uses water as a solvent, it is a one-bottle system and the more complex chemistry of this system may make it more susceptible to the contamination procedure.
Under the S condition, the adhesive/dentin interfacial microstructures of these two bonding agents were very similar to their respective control groups. These results support the observation of a high affinity between the two-bottle self-etch adhesive system chemistry of SEB and moist conditions.
SBP results for the S condition were severely reduced, but with conditions SA and SW the values were reduced by about 10% so that this adhesive was less sensitive to the artificial saliva contamination with and without mucin. Rinsing (SW) did not restore the bond strength to the values of the control, but also did not further decrease the bond strength. The inclusion of mucin in the saliva did not have a substantial effect on this system. The SEM analysis showed that the adhesive/dentin interface of this bonding agent with this condition was very similar to the control group.
Dried artificial saliva resulted in decreased bond strength for P&BNT, but when the contaminated surface was washed (SW) bond strengths increased to levels greater than 20 MPa. Although it is recommended by the manufacturer to work under moist conditions with this adhesive system, dry conditions were used in this study under some of the contaminated conditions. The dry conditions could lead to collagen collapse [32–34, 39]. The SEM analysis showed a very similar structure to the S condition with a thick hybrid layer, long and numerous resin tags but cracks throughout the adhesive/dentin interface structure. The cracked microstructure may be one explanation for bond strengths that are close to 20 MPa, but only one-half to one-third the bond strength values were recorded with the control specimens.
SEB results with SA and SW conditions showed a decrease in bond strength but were not reduced to values as low as P&BNT with SA condition. In fact, contaminated and washed (SW) conditions gave bond strengths of about 20 MPa with mucin, but without mucin the bond strength results were 10 MPa lower. The SEM analysis did not show identifiable changes in the adhesive/dentin interface microstructure between SW and S conditions. Thus, further analysis of the dentin/adhesive interface should be undertaken to help determine the quality and molecular structure of the interfaces under conditions such as wet bonding [28].
S3 results with SA conditions showed a significant decrease in the bond strength with and without mucin to values lower than 20 MPa. However, with the SW condition the results were similar to controls for saliva without mucin and had higher values when the saliva contained mucin as compared to the control group. The saliva pellicle components may have affected the bonding process, but it is surprising that the saliva components that affected the mechanical properties were not washed away after rinsing the dentin for 5 s. SEM analysis of S3 under SA condition showed the longest resin tags for this bonding agent compared with the other conditions. These results potentially reflect the hydrophobic nature of this bonding agent and the manufacturer’s recommendation to use dry bonding conditions with this adhesive. Additional property measurements and analyses with other technologies are required to adequately explain the phenomenon contributing to the differences noted in this study.
The null hypotheses of this study were rejected, with some exceptions, because there were significant differences in bond strength among adhesive systems contaminated with artificial saliva with and without mucin and under some of the conditions. Both etch and rinse systems were sensitive to contamination, while SEB was not very sensitive to salivary contamination (S), but showed decreased bond strengths for both SA and SW conditions. Furthermore, there were significant differences in dentin-adhesive interfacial morphology among adhesive systems using saliva with and without mucin under different conditions of decontaminating the interface. In general, both etch and rinse systems (SBP and P&BNT) were tremendously affected (0 MPa) after contamination with S condition. On the other hand, both self-etch systems presented mixed (particularly S and SW with SEB and SA and SW with S3 increased with mucin) bond strength results.
Conclusions
Based on the results of this in vitro study the following conclusions were made:
P&BNT (control) following the manufacturer’s instructions had the highest bond strengths and the dentin/adhesive interface exhibited a standard morphology characteristic of etch-and-rinse system.
It is not possible to conclude that the 450 mg/L mucin added to our artificial saliva model was responsible for bond strength results, as the addition of mucin did not have dramatic effects on bond strength. This model contains several other components that might be responsible for the mixed results. Future studies should evaluate the effect of other proteins and protein concentrations as well as other saliva components in the adhesion of bonding agents to tooth structures.
Acknowledgments
The authors wish to acknowledge UMKCCRISP and the Electron Microscopy Laboratory in the Department of Oral Biology at the UMKC School of Dentistry, Denstply/Caulk, 3MESPE, Kuraray America, NIH/NIDCR T32 DE07204 and T32 DE07306, and the Biomaterials Research Center at the University of Texas-Houston for supporting this study, Larry Watanabe, Clarice Thenard from UCSF School of Dentistry.
Footnotes
Presented at the Annual Meeting of the American Association for Dental Research, Orlando, Florida, USA, March 2006.
Contributor Information
Lilliam Marie Pinzon, Email: Lilliam.Pinzon@ucsf.edu, Department of Preventive and Restorative Dentistry, University of California, San Francisco, 707 Parnassus Avenue, Room 3212, San Francisco, CA 94143-0758, USA.
John M. Powers, Department of Restorative Dentistry and Biomaterials, The University of Texas Dental Branch at Houston, Houston, TX, USA
Kathy L. O’Keefe, Department of Restorative Dentistry and Biomaterials, The University of Texas Dental Branch at Houston, Houston, TX, USA
Vladimir Dusevish, The University of Missouri, Kansas City, MI, USA.
Paulette Spencer, Department of Mechanical Engineering, The University of Kansas, Lawrence, KS, USA.
Grayson W. Marshall, Division of Biomaterials and Bioengineering, Department of Preventive and Restorative Dental Sciences, The University of California, San Francisco, San Francisco, CA, USA
References
- 1.Eick JD, Gwinnett AJ, Pashley DH, Robinson SJ. Current concepts on adhesion to dentin. Crit Rev Oral Biol Med. 1997;8:306–335. doi: 10.1177/10454411970080030501. [DOI] [PubMed] [Google Scholar]
- 2.Gordan VV, Vargas MA, Cobb DS, Denehy GE. Evaluation of adhesive systems using acidic primers. Am J Dent. 1997;10:219–223. [PubMed] [Google Scholar]
- 3.Safar JA, Davis RD, Overton JD. Effect of saliva contamination on the bond of dentin to resin-modified glass-ionomer cement. Oper Dent. 1999;24:351–357. [PubMed] [Google Scholar]
- 4.Powers JM, Finger WJ, Xie J. Bonding of composite resin to contaminated human enamel and dentin. J Prosthodont. 1995;4:28–32. doi: 10.1111/j.1532-849x.1995.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 5.Yoo HM, Oh TS, Pereira PN. Effect of saliva contamination on the microshear bond strength of one self-etching adhesive systems to dentin. Oper Dent. 2006;31:127–134. doi: 10.2341/04-206. [DOI] [PubMed] [Google Scholar]
- 6.Sattabanasuk V, Shimada Y, Tagami J. Effects of saliva contamination on dentin bond strength using all-in-one adhesives. J Adhes Dent. 2006;8:311–318. [PubMed] [Google Scholar]
- 7.Pashley EL, Tao L, Mackert JR, Pashley DH. Comparison of in vivo vs. in vitro bonding of composite resin to the dentin of canine teeth. J Dent Res. 1988;67:467–470. doi: 10.1177/00220345880670020601. [DOI] [PubMed] [Google Scholar]
- 8.Van Schalkwyk JH, Botha FS, van der Vyver PJ, de Wet FA, Botha SJ. Effect of biological contamination on dentin bond strength of adhesive resins. SADJ. 2003;58:143–147. [PubMed] [Google Scholar]
- 9.Ellison S. Handbook of physiology. Baltimore: Williams and Wilkins Co; 1967. Proteins and glycoproteins of saliva. [Google Scholar]
- 10.van Nieuw Amerongen A, Bolscher JG, Veerman EC. Salivary proteins: protective and diagnostic value in cariology. Caries Res. 2004;38:247–253. doi: 10.1159/000077762. [DOI] [PubMed] [Google Scholar]
- 11.Tabak LA. In defense of the oral cavity: the protective role of salivary secretions. Pediatr Dent. 2006;28:110–117. [PubMed] [Google Scholar]
- 12.Wolf RO, Taylor LL, Niswander JD, Schwartz JT. The heritability of human salivary isoamylases. Arch Oral Biol. 1971;16:1357–1359. doi: 10.1016/0003-9969(71)90037-9. [DOI] [PubMed] [Google Scholar]
- 13.Tabak LA, Levine MJ, Mandel ID, Ellison SA. Role of salivary mucins in the protection of the oral cavity. J Oral Pathol. 1982;11:1–17. doi: 10.1111/j.1600-0714.1982.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 14.Duarte SJ, Lolato AL, de Freitas CR, Dinelli W. SEM analysis of internal adaptation of adhesive restorations after contamination with saliva. J Adhes Dent. 2005;7:51–56. [PubMed] [Google Scholar]
- 15.Park JW, Lee KC. The influence of salivary contamination on shear bond strength of dentin adhesive systems. Oper Dent. 2004;29:437–442. [PubMed] [Google Scholar]
- 16.Fritz UB, Finger WJ, Stean H. Salivary contamination during bonding procedures with a one-bottle adhesive system. Quintessence Int. 1998;29:567–572. [PubMed] [Google Scholar]
- 17.Nascimento ABL, Reis JIL, Fontana U. Effects of saliva contamination on shear bond strengths to dentin. J Dent Res. 1997;76:424. [Google Scholar]
- 18.el-Kalla IH, Garcia-Godoy F. Saliva contamination and bond strength of single-bottle adhesives to enamel and dentin. Am J Dent. 1997;10:83–87. [PubMed] [Google Scholar]
- 19.Abdalla AI, Davidson CL. Bonding efficiency and interfacial morphology of one-bottle adhesives to contaminated dentin surfaces. Am J Dent. 1998;11:281–285. [PubMed] [Google Scholar]
- 20.Jacobsen T, Soderholm KJ. Some effects of water on dentin bonding. Dent Mater. 1995;11:132–136. doi: 10.1016/0109-5641(95)80048-4. [DOI] [PubMed] [Google Scholar]
- 21.Tao L, Pashley DH. Dentin Perfusion Effects on the Shear Bond Strengths of Bonding Agents to Dentin. Dent Mater. 1989;5:181–184. doi: 10.1016/0109-5641(89)90010-9. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Spencer P, Yao X, Ye Q. Effect of co-initiator and water on the photoreactivity and photopolymerization of HEMA/camphoroquinone-based reactant mixtures. J Biomed Mater Res A. 2006;78(4):721–728. doi: 10.1002/jbm.a.30733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bishara SE, Oonsombat C, Ajlouni R, Denehy G. The effect of saliva contamination on shear bond strength of orthodontic brackets when using a self-etch primer. Angle Orthod. 2002;72:554–557. doi: 10.1043/0003-3219(2002)072<0554:TEOSCO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Schaneveldt S, Foley TF. Bond strength comparison of moisture-insensitive primers. Am J Orthod Dentofac Orthop. 2002;122:267–273. doi: 10.1067/mod.2002.126594. [DOI] [PubMed] [Google Scholar]
- 25.Perdigao J, Frankenberger R. Effect of solvent and rewetting time on dentin adhesion. Quintessence Int. 2001;32:385–390. [PubMed] [Google Scholar]
- 26.Hitmi L, Attal JP, Degrange M. Influence of the time-point of salivary contamination on dentin shear bond strength of 3 dentin adhesive systems. J Adhes Dent. 1999;1:219–232. [PubMed] [Google Scholar]
- 27.Eiriksson SO, Pereira PN, Swift EJ, Jr, Heymann HO, Sigurdsson A. Effects of saliva contamination on resin-resin bond strength. Dent Mater. 2004;20:37–44. doi: 10.1016/s0109-5641(03)00066-6. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Spencer P. Hybridization efficiency of the adhesive/dentin interface with wet bonding. J Dent Res. 2003;82:141–145. doi: 10.1177/154405910308200213. [DOI] [PubMed] [Google Scholar]
- 29.Nakabayashi N, Ashizawa M, Nakamura M. Identification of a resin-dentin hybrid layer in vital human dentin created in vivo: durable bonding to vital dentin. Quintessence Int. 1992;23:135–141. [PubMed] [Google Scholar]
- 30.Pinzon LMSE, Reis AF, Watanabe LG, Giannini M, Powers JM, Tomsi AP, Marshall SJ, Marshall GW. Interfacial structure and bond strengths of self-etch adhesive systems. J Dent Res. 2006 (Abstract 2552) [PMC free article] [PubMed] [Google Scholar]
- 31.Hormati AA, Fuller JL, Denehy GE. Effects of contamination and mechanical disturbance on the quality of acid-etched enamel. J Am Dent Assoc. 1980;100:34–38. doi: 10.14219/jada.archive.1980.0033. [DOI] [PubMed] [Google Scholar]
- 32.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res. 2002;62:447–456. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 33.Katz JL, Bumrerraj S, Dreyfuss J, Wang Y, Spencer P. Micromechanics of the dentin/adhesive interface. J Biomed Mater Res. 2001;58:366–371. doi: 10.1002/jbm.1030. [DOI] [PubMed] [Google Scholar]
- 34.Gwinnett AJ. Dentin bond strength after air drying and rewetting. Am J Dent. 1994;7:144–148. [PubMed] [Google Scholar]
- 35.Spencer P, Katz JL, Tabbib-Azar M, Wang Y, Wagh A, Nomura T. Hyperspectral analysis of collagen infused with BisGMA-based polymeric adhesive. In: Yaszensju MJ, Trantolo DJ, Lewandrowski KU, Hasirci V, Altobelli DE, Wise DL, editors. Tissue engineering and novel delivery systems. New York: Marcel Decker; 2003. pp. 599–632. [Google Scholar]
- 36.Wang Y, Spencer P. Quantifying adhesive penetration in adhesive/dentin interface using confocal Raman microspectroscopy. J Biomed Mater Res. 2002;59:46–55. doi: 10.1002/jbm.1215. [DOI] [PubMed] [Google Scholar]
- 37.Ye Q, Park J, Topp E, Spencer P. Effect of photoinitiators on the in vitro performance of a dentin adhesive exposed to simulated oral environment. Dent Mater. 2009;25:452–48. doi: 10.1016/j.dental.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye Q, Spencer P, Wang Y, Misra A. Relationship of solvent to photopolymerization process, properties, and structure in model dentin adhesives. J Biomed Mater Res. 2008;80:342–350. doi: 10.1002/jbm.a.30890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gwinnet AJ. Moist versus dry dentin: its effect on shear bond strength. Am J Dent. 1992;5:127–129. [PubMed] [Google Scholar]